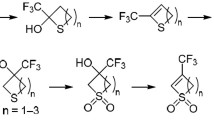
The reactions of α,β-unsaturated ketones and Lawesson’s reagent with maleic, itaconic, or 5-norbornene-2,3-dicarboxylic acid led to the corresponding 3,4-dihydro-2H-thiopyran anhydrides. As a result of the reaction of methacrylic acid, Lawesson’s reagent, and chalcone, 4-methyl-1,8-diphenyl-2,6-dithiabicyclo[2.2.2]octan-3-one is formed. The selectivity of the acylation of amines using the synthesized anhydrides was studied.



Similar content being viewed by others
References
(a) Antipin, I. S.; Kazymova, M. A.; Kuznetsov, M. A.; Vasilyev, A. V.; Ishchenko, M. A.; Kiryushkin, A. A.; Kuznetsova, L. M.; Makarenko, S. V.; Ostrovskii, V. A.; Petrov, M. L.; Solod, O. V.; Trishin, Yu. G.; Yakovlev, I. P.; Nenajdenko, V. G.; Beloglazkina, E. K.; Beletskaya, I. P.; Ustynyuk, Yu. A.; Solov’ev, P. A.; Ivanov, I. V.; Malina, E. V.; Sivova, N. V.; Negrebetskii, V. V.; Baukov, Yu. I.; Pozharskaya, N. A.; Traven’, V. F.; Shchekotikhin, A. E.; Varlamov, A. V.; Borisova, T. N.; Lesina, Yu. A.; Krasnokutskaya, E. A.; Rogozhnikov, S. I.; Shurov, S. N.; Kustova, T. P.; Klyuev, M. V.; Khelevina, O. G.; Stuzhin, P. A.; Fedorov, A. Yu.; Gushchin, A. V.; Dodonov, V. A.; Kolobov, A. V.; Plakhtinskii, V. V.; Orlov, V. Yu.; Kriven’ko, A. P.; Fedotova, O. V.; Pchelintseva, N. V.; Charushin, V. N.; Chupakhin, O. N.; Klimochkin, Yu. N.; Klimochkina, A. Yu.; Kuryatnikov, V. N.; Malinovskaya, Yu. A.; Levina, A. S.; Zhuravlev, O. E.; Voronchikhina, L. I.; Fisyuk, A. S.; Aksenov, A. V.; Aksenov, N. A.; Aksenova, I. V. Russ. J. Org. Chem. 2017, 53, 1275. [Zh. Org. Khim. 2017, 53, 1257.] (b) Konovalov, A. I.; Antipin, I. S.; Burilov, V. A.; Madzhidov, T. I.; Kurbangalieva, A. R.; Nemtarev, A. V.; Solovieva, S. E.; Stoikov, I. I.; Mamedov, V. A.; Zakharova, L. Ya.; Gavrilova, E. L.; Sinyashin, O. G.; Balova, I. A.; Vasilyev, A. V.; Zenkevich, I. G.; Krasavin, M. Yu.; Kuznetsov, M. A.; Molchanov, A. P.; Novikov, M. S.; Nikolaev, V. A.; Rodina, L. L.; Khlebnikov, A. F.; Beletskaya, I. P.; Vatsadze, S. Z.; Gromov, S. P.; Zyk, N. V.; Lebedev, A. T.; Lemenovskii, D. A.; Petrosyan, V. S.; Nenaidenko, V. G.; Negrebetskii, V. V.; Baukov, Yu. I.; Shmigol’, T. A.; Korlyukov, A. A.; Tikhomirov, A. S.; Shchekotikhin, A. E.; Traven’, V. F.; Voskresenskii, L. G.; Zubkov, F. I.; Golubchikov, O. A.; Semeikin, A. S.; Berezin, D. B.; Stuzhin, P. A.; Filimonov, V. D.; Krasnokutskaya, E. A.; Fedorov, A. Yu.; Nyuchev, A. V.; Orlov, V. Yu.; Begunov, R. S.; Rusakov, A. I.; Kolobov, A. V.; Kofanov, E. R.; Fedotova, O. V.; Egorova, A. Yu.; Charushin, V. N.; Chupakhin, O. N.; Klimochkin, Yu. N.; Osyanin, V. A.; Reznikov, A. N.; Fisyuk, A. S.; Sagitullina, G. P.; Aksenov, A. V.; Aksenov, N. A.; Grachev, M. K.; Maslennikova, V. I.; Koroteev, M. P.; Brel’, A. K.; Lisina, S. V.; Medvedeva, S. M.; Shikhaliev, Kh. S.; Suboch, G. A.; Tovbis, M. S.; Mironovich, L. M.; Ivanov, S. M.; Kurbatov, S. V.; Kletskii, M. E.; Burov, O. N.; Kobrakov, K. I.; Kuznetsov, D. N. Russ. J. Org. Chem. 2018, 54, 157. [Zh. Org. Khim. 2018, 54, 161.]
(a) Merkulova, Е. А.; Kolobov, А. V.; Ovchinnikov, K. L. RF patent № 2670977; Byull. Izobret. 2018, (30). (b) Merkulova, E. A.; Kolobov, A. V.; Ovchinnikov, K. L. Russ. Chem. Bull., Int. Ed. 2019, 68, 606. [Izv. Akad. Nauk, Ser. Khim. 2019, 606.]
Ozturk, T.; Ertas, E.; Mert, O. Chem. Rev. 2007, 107, 5210.
(a) Karakasa, T.; Motoki, S. J. Org. Chem. 1979, 44, 4151. (b) Karakasa, T.; Motoki, S. J. Org. Chem. 1978, 43, 4147.
Bogdanowicz-Szwed, K.; Budzowski, A. Z. Naturforschung, B: J. Chem. Sci. 2002, 57, 637.
Rao, Y.; Li, X.; Nagorny, P.; Hayashida, J.; Danishefsky, S. J. Tetrahedron Lett. 2009, 50, 6684.
(a) Scheibye, S.; Shabana, R.; Lawesson, S.-O.; Rømming, C. Tetrahedron 1982, 38, 993. (b) Przychodzeń, W. Eur. J. Org. Chem. 2005, 10, 2002.
Kolobov, А. V. Doctoral (Chem.) Dissertation; Yaroslavl, 2007. https://www.dissercat.com/content/vitsinalnye-dikarbonovyekisloty-sintez-struktura-svoistva
Gordon, А.; Ford, R. Sputnik khimika (The Chemist’s Companion [Russian translation]); Moscow: Mir, 1976, p. 301.
Cinar, S. A.; Ercan, S.; Gunal, S. E.; Dogan I.; Aviyente, V. Org. Biomol. Chem. 2014, 12, 8079.
Cava, M. P.; Levinson, M. I. Tetrahedron 1985, 41, 5061.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
(a) Palatinus, L.; Chapuis, G. J. Appl. Crystallogr. 2007, 40, 786. (b) Palatinus, L.; Van der Lee, A. J. Appl. Crystallogr. 2008, 41, 975. (c) Palatinus, L.; Prathapa, S. J.; van Smaalen, S. J. Appl. Crystallogr. 2012, 45, 575.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(3), 245–252
Rights and permissions
About this article
Cite this article
Merkulova, E.А., Kolobov, A.V., Ovchinnikov, K.L. et al. Unsaturated carboxylic acids in the one-pot synthesis of novel derivatives of 3,4-dihydro-2H-thiopyran. Chem Heterocycl Comp 57, 245–252 (2021). https://doi.org/10.1007/s10593-021-02900-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02900-y