Abstract
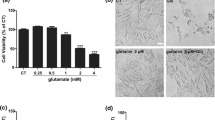
Oxidative glutamate toxicity is involved in diverse neurological disorders including epilepsy and ischemic stroke. Our present work aimed to assess protective effects of huperzine A (HupA) against oxidative glutamate toxicity in a mouse-derived hippocampal HT22 cells and explore its potential mechanisms. Cell survival and cell injury were analyzed by MTT method and LDH release assay, respectively. The production of ROS was measured by detection kits. Protein expressions of BDNF, phosphor-TrkB (p-TrkB), TrkB, phosphor-Akt (p-Akt), Akt, phosphor-mTOR (p-mTOR), mTOR, phosphor-p70s6 (p-p70s6) kinase, p70s6 kinase, Bcl-2, Bax, and β-actin were assayed via Western blot analysis. Enzyme-linked immunosorbent assay was employed to measure the contents of nerve growth factor, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). Our findings illustrated 10 μM HupA for 24 h significantly protected HT22 from cellular damage and suppressed the generation of ROS. Additionally, after treating with LY294002 or wortmannin [the selective inhibitors of phosphatidylinositol 3 kinase (PI3K)], HupA dramatically prevented the down-regulations of p-Akt, p-mTOR, and p-p70s6 kinase in HT22 cells under oxidative toxicity. Furthermore, it was observed that the protein levels of BDNF and p-TrkB were evidently enhanced after co-treatment with HupA and glutamate in HT22 cells. The elevations of p-Akt and p-mTOR were abrogated under toxic conditions after blockade of TrkB by TrkB IgG. Cellular apoptosis was significantly suppressed (decreased caspase-3 activity and enhanced Bcl-2 protein level) after HupA treatment. It was concluded that HupA attenuated oxidative glutamate toxicity in murine hippocampal HT22 cells via activating BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway.







Similar content being viewed by others
References
Ahn SM et al (2015) Neuroprotection and spatial memory enhancement of four herbal mixture extract in HT22 hippocampal cells and a mouse model of focal cerebral ischemia. BMC Complement Altern Med 15:202. doi:10.1186/s12906-015-0741-1
Almeida RD et al (2005) Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12:1329–1343. doi:10.1038/sj.cdd.4401662
Bao MH, Zhang YW, Zhou HH (2013) Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-kappaB pathway. J Ethnopharmacol 146:543–551. doi:10.1016/j.jep.2013.01.019
Beck T, Lindholm D, Castren E, Wree A (1994) Brain-derived neurotrophic factor protects against ischemic cell damage in rat hippocampus. J Cereb Blood Flow Metab 14:689–692. doi:10.1038/jcbfm.1994.86
Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11:297–305
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Han YJ et al (2014) Gastrodia elata shows neuroprotective effects via activation of PI3K signaling against oxidative glutamate toxicity in HT22 cells. Am J Chin Med 42:1007–1019. doi:10.1142/s0192415x14500633
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642. doi:10.1146/annurev.biochem.72.121801.161629
Jackson GR, Apffel L, Werrbach-Perez K, Perez-Polo JR (1990) Role of nerve growth factor in oxidant-antioxidant balance and neuronal injury. I. Stimulation of hydrogen peroxide resistance. J Neurosci Res 25:360–368. doi:10.1002/jnr.490250313
Jeong GS, Byun E, Li B, Lee DS, Kim YC, An RB (2010) Neuroprotective effects of constituents of the root bark of Dictamnus dasycarpus in mouse hippocampal cells. Arch Pharm Res 33:1269–1275. doi:10.1007/s12272-010-0818-9
Jia J et al (2013) Differential mechanisms underlying neuroprotection of hydrogen sulfide donors against oxidative stress. Neurochem Int 62:1072–1078. doi:10.1016/j.neuint.2013.04.001
Kazanis I, Giannakopoulou M, Philippidis H, Stylianopoulou F (2004) Alterations in IGF-I, BDNF and NT-3 levels following experimental brain trauma and the effect of IGF-I administration. Exp Neurol 186:221–234. doi:10.1016/j.expneurol.2003.12.004
Larsson E, Nanobashvili A, Kokaia Z, Lindvall O (1999) Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J Cereb Blood Flow Metab 19:1220–1228. doi:10.1097/00004647-199911000-00006
Lee DS, Cha BY, Woo JT, Kim YC, Jang JH (2015) Acerogenin A from nikoense maxim prevents oxidative stress-induced neuronal cell death through Acer Nrf2-mediated heme oxygenase-1 expression in mouse hippocampal HT22 Cell Line. Molecules 20:12545–12557. doi:10.3390/molecules200712545
Mao X et al (2012) Topiramate attenuates cerebral ischemia/reperfusion injury in gerbils via activating GABAergic signaling and inhibiting astrogliosis. Neurochem Int 60:39–46. doi:10.1016/j.neuint.2011.10.015
Mao XY, Cao YG, Ji Z, Zhou HH, Liu ZQ, Sun HL (2015) Topiramate protects against glutamate excitotoxicity via activating BDNF/TrkB-dependent ERK pathway in rodent hippocampal neurons. Prog Neuropsychopharmacol Biol Psychiatry 60:11–17. doi:10.1016/j.pnpbp.2015.01.015
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1:120–129. doi:10.1038/35040009
Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2:1547–1558
Shukry M, Kamal T, Ali R, Farrag F, Almadaly E, Saleh AA, Abu El-Magd M (2015) Pinacidil and levamisole prevent glutamate-induced death of hippocampal neuronal cells through reducing ROS production. Neurol Res. doi:10.1179/1743132815y.0000000077
Simonian NA, Coyle JT (1996) Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 36:83–106. doi:10.1146/annurev.pa.36.040196.000503
Tang LL, Wang R, Tang XC (2005) Huperzine A protects SHSY5Y neuroblastoma cells against oxidative stress damage via nerve growth factor production. Eur J Pharmacol 519:9–15. doi:10.1016/j.ejphar.2005.06.026
Wang ZF, Tang XC (2007) Huperzine A protects C6 rat glioma cells against oxygen-glucose deprivation-induced injury. FEBS Lett 581:596–602. doi:10.1016/j.febslet.2007.01.016
Wang R, Xiao XQ, Tang XC (2001) Huperzine A attenuates hydrogen peroxide-induced apoptosis by regulating expression of apoptosis-related genes in rat PC12 cells. NeuroReport 12:2629–2634
Wang R, Yan H, Tang XC (2006) Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin 27:1–26. doi:10.1111/j.1745-7254.2006.00255.x
Wang R et al (2010) Curcumin produces neuroprotective effects via activating brain-derived neurotrophic factor/TrkB-dependent MAPK and PI-3K cascades in rodent cortical neurons. Prog Neuropsychopharmacol Biol Psychiatry 34:147–153. doi:10.1016/j.pnpbp.2009.10.016
Wang CY et al (2011) Huperzine A activates Wnt/beta-catenin signaling and enhances the nonamyloidogenic pathway in an Alzheimer transgenic mouse model. Neuropsychopharmacology 36:1073–1089. doi:10.1038/npp.2010.245
Yang EJ et al (2012) Isoliquiritigenin isolated from Glycyrrhiza uralensis protects neuronal cells against glutamate-induced mitochondrial dysfunction. Biochem Biophys Res Commun 421:658–664. doi:10.1016/j.bbrc.2012.04.053
Yang EJ, Kim GS, Jun M, Song KS (2014) Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct 5:1395–1402. doi:10.1039/c4fo00068d
Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX (2012) Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res 37:2777–2786. doi:10.1007/s11064-012-0871-5
Zhou J, Zhang HY, Tang XC (2001) Huperzine A attenuates cognitive deficits and hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci Lett 313:137–140
Acknowledgments
This project was partly funded by the National Natural Science Foundation of China (No. 81302750).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
These authors declared no potential conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10571_2015_276_MOESM1_ESM.tiff
Supplementary material 1 S-Fig. 1 HupA attenuated neuronal impairment in human neuroblastoma SH-SY5Y cells under glutamate stimulation. SH-SY5Y cells was subjected to different concentrations of glutamate (2, 4, 6, 8 and 10 mM) for 24 h and cell viability was measured by MTT method (a). Nearly a 30% reduction of cell viability was found after glutamate exposure at the concentration of 10 mM for 24 h. HupA by the concentrations of 0-10 μM did not have any significant toxic effects on the cell viability of SH-SY5Y cells (b). It was also noted that 10 μM of HupA treatment for 24 h evidently promoted the recovery of SH-SY5Y cells after glutamate exposure (c). Results were expressed as mean ± standard deviation (SD) (n = 8). ** p<0.01 versus control group; ## p<0.01 versus glutamate-treated group. (TIFF 306 kb)
Rights and permissions
About this article
Cite this article
Mao, XY., Zhou, HH., Li, X. et al. Huperzine A Alleviates Oxidative Glutamate Toxicity in Hippocampal HT22 Cells via Activating BDNF/TrkB-Dependent PI3K/Akt/mTOR Signaling Pathway. Cell Mol Neurobiol 36, 915–925 (2016). https://doi.org/10.1007/s10571-015-0276-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0276-5