Abstract
Purpose
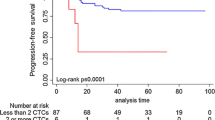
Several studies have provided evidence on the prognostic relevance of circulating tumor cells (CTCs) detected before and after chemotherapy regarding overall survival (OS) and progression-free survival (PFS) in early breast cancer (EBC). We provide data on the prevalence of CTCs 2 and 5 years after primary diagnosis in a cohort of patients with EBC.
Methods
The SUCCESS study is a multicenter, prospective, randomized trial comparing PFS in primary breast cancer patients undergoing one of two adjuvant chemotherapy regimens followed by 2 versus 5 years of treatment with zoledronate. CTCs from patients without signs of breast cancer recurrence were analyzed in peripheral blood using the FDA cleared CellSearch® System (Veridex, USA) 2 and 5 years after primary diagnosis.
Results
CTCs were detected at 2 and 5 years after primary diagnosis in 96 (16.7%) and 47 (8.2%) of the 574 patients, respectively. There were no associations between CTC status and patient and tumor characteristics or treatment regimens. In 442 (77.0%) patients, no CTCs were detected at either of the two time points, and in 11 patients (1.9%), CTCs were found at both 2 and 5 years after primary diagnosis. In 85 (14.8%) patients, CTCs were present 2 years after primary diagnosis but not after 5 years, while 36 (6.3%) patients had CTCs in their blood only at the 5-year follow-up.
Conclusions
In patients with EBC, CTCs can be detected even 5 years after primary diagnosis without clinical signs of disease recurrence.



Similar content being viewed by others
References
Lu WL, Jansen L, Post WJ, Bonnema J, van de Velde JC, de Bock GH (2009) Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: a meta-analysis. Breast Cancer Res Treat 114:403–412. https://doi.org/10.1007/s10549-008-0023-4
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351:781–791. https://doi.org/10.1056/NEJMoa040766
Bidard FC, Peeters DJ, Fehm T et al (2014) Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 15:406–414. https://doi.org/10.1016/s1470-2045(14)70069-5
Rack B, Schindlbeck C, Juckstock J et al (2014) Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju066
Janni W, Rack B, Terstappen LW et al (2016) Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res 22:2583–2593. https://doi.org/10.1158/1078-0432.ccr-15-1603
Janni W, Rack B, Fasching P, Haeberle L, Friedl T, Tesch H, Lorenz R, Neugebauer J, Koch J, Jaeger B (2016) Abstract S2-03: persistence of circulating tumor cells in high risk early breast cancer patients during follow-up care suggests poor prognosis—results from the adjuvant SUCCESS A trial. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS15-S2-03
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10:6897–6904. https://doi.org/10.1158/1078-0432.ccr-04-0378
Riethdorf S, Fritsche H, Muller V et al (2007) Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the cellsearch system. Clin Cancer Res 13:920–928. https://doi.org/10.1158/1078-0432.ccr-06-1695
Alunni-Fabbroni M, Sandri MT (2010) Circulating tumour cells in clinical practice: methods of detection and possible characterization. Methods 50:289–297. https://doi.org/10.1016/j.ymeth.2010.01.027
Franken B, de Groot MR, Mastboom WJ, Vermes I, van der Palen J, Tibbe AG, Terstappen LW (2012) Circulating tumor cells, disease recurrence and survival in newly diagnosed breast cancer. Breast Cancer Res 14:R133. https://doi.org/10.1186/bcr3333
Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S (2012) Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 13:688–695. https://doi.org/10.1016/s1470-2045(12)70209-7
Ignatiadis M, Xenidis N, Perraki M, Apostolaki S, Politaki E, Kafousi M, Stathopoulos EN, Stathopoulou A, Lianidou E, Chlouverakis G, Sotiriou C, Georgoulias V, Mavroudis D (2007) Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol 25:5194–5202. https://doi.org/10.1200/jco.2007.11.7762
Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, Marty M, Pierga JY (2010) Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol 21:729–733. https://doi.org/10.1093/annonc/mdp391
Meng S, Tripathy D, Frenkel EP et al (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10:8152–8162. https://doi.org/10.1158/1078-0432.ccr-04-1110
Sparano J, O’Neill A, Alpaugh K, Wolff AC, Northfelt DW, Dang C, Sledge GW Jr, Miller KD (2018) Abstract GS6-03: circulating tumor cells (CTCs) five years after diagnosis are prognostic for late recurrence in operable stage II-III breast cancer. Cancer Res doi. https://doi.org/10.1158/1538-7445.SABCS17-GS6-03
Pachmann K, Camara O, Kavallaris A et al (2008) Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol 26:1208–1215. https://doi.org/10.1200/jco.2007.13.6523
Sandri MT, Zorzino L, Cassatella MC, Bassi F, Luini A, Casadio C, Botteri E, Rotmensz N, Adamoli L, Nole F (2010) Changes in circulating tumor cell detection in patients with localized breast cancer before and after surgery. Ann Surg Oncol 17:1539–1545. https://doi.org/10.1245/s10434-010-0918-2
Wulfing P, Borchard J, Buerger H, Heidl S, Zanker KS, Kiesel L, Brandt B (2006) HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res 12:1715–1720. https://doi.org/10.1158/1078-0432.ccr-05-2087
Ignatiadis M, Perraki M, Apostolaki S, Politaki E, Xenidis N, Kafousi M, Stathopoulos E, Lianidou E, Sotiriou C, Georgoulias V, Mavroudis D (2007) Molecular detection and prognostic value of circulating cytokeratin-19 messenger RNA-positive and HER2 messenger RNA-positive cells in the peripheral blood of women with early-stage breast cancer. Clin Breast Cancer 7:883–889. https://doi.org/10.3816/CBC.2007.n.054
Lang JE, Mosalpuria K, Cristofanilli M, Krishnamurthy S, Reuben J, Singh B, Bedrosian I, Meric-Bernstam F, Lucci A (2009) HER2 status predicts the presence of circulating tumor cells in patients with operable breast cancer. Breast Cancer Res Treat 113:501–507. https://doi.org/10.1007/s10549-008-9951-2
Gaforio JJ, Serrano MJ, Sanchez-Rovira P, Sirvent A, Delgado-Rodriguez M, Campos M, de la Torre N, Algarra I, Duenas R, Lozano A (2003) Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer 107:984–990. https://doi.org/10.1002/ijc.11479
Funding
This translational research portion of the SUCCESS A trial has been supported by AstraZeneca, Chugai, Lilly, Novartis, Sanofi-Aventis, and Veridex.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
B. Rack, A. Schneeweiss, T. Fehm, M. W. Beckmann, and W. Janni have received research funding from AstraZeneca, Chugai, Lilly, Novartis, and Sanofi-Aventis. B. Rack, T. Fehm, K. Pantel, and W. Janni received research funding and speaker honoraria from Veridex. M. W. Beckmann acted as advisors for Novartis and Sanofi-Aventis. A. Schneeweiss received speaker honoraria from AstraZeneca, Chugai, Lilly, Novartis, and Sanofi-Aventis. C. P. A. Fasching received research funding and speaker honoraria from Novartis. E. C.A. Bauer, F. Schochter, P. Widschwendter, A. DeGregorio, T.W.P. Friedl, and C. Scholz have no conflict of interest to declare.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration, its later amendments, and comparable ethical standards.
Informed consent
Written informed consent was obtained from all study participants prior to study inclusion.
Rights and permissions
About this article
Cite this article
Bauer, E.C.A., Schochter, F., Widschwendter, P. et al. Prevalence of circulating tumor cells in early breast cancer patients 2 and 5 years after adjuvant treatment. Breast Cancer Res Treat 171, 571–580 (2018). https://doi.org/10.1007/s10549-018-4856-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4856-1