Abstract
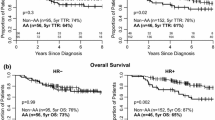
Previous studies demonstrated poor response to neoadjuvant systemic therapy (NST) for breast cancer among black women and women who are overweight or obese, but this may be due to chemotherapy underdosing. We assessed associations of race, ethnicity, and body mass index (BMI) with pathologic complete response (pCR) in clinical trial populations. 1797 women enrolled in four NST trials (CALGB 40601, 40603; ACOSOG Z1041, Z1071) were included. Tumor subtypes were defined by estrogen receptor (ER) and HER2 status. Logistic regression generated odds ratios (OR) and 95 % confidence intervals (CI) for the associations of race, ethnicity, and BMI with in-breast pCR adjusting for subtype, study arm, lymph node status, tumor size, and tumor grade. 253 (14.1 %) were black, 199 (11.1 %) Hispanic, 520 (28.9 %) overweight, and 743 (41.4 %) obese. Compared to whites, Blacks and Hispanics were more likely to be obese and Blacks were more likely to have triple-negative cancer. pCR rates differed significantly by tumor subtype. In multivariate analyses, neither race (black vs white: OR 1.18, 95 % CI 0.85–1.62) nor ethnicity (Hispanic vs non-Hispanic; OR 1.30, 95 % CI 0.67–2.53) were significant predictors of pCR overall or by subtype. Overweight and obese women had lower pCR rates in ER+/HER2+, but higher pCR rates in ER−/HER2+ cancers. There was no difference in pCR according to race or ethnicity. Overall, there was no major difference in pCR rates by BMI. These findings suggest that pCR with optimally dosed NST is a function of tumor, rather than patient, biology.
Similar content being viewed by others
References
Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Fetting J, Davidson NE (2012) Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. doi:10.1002/cncr.27527
Ligibel JA, Cirrincione CT, Liu M, Citron M, Ingle JN, Gradishar W, Martino S, Sikov W, Michaelson R, Mardis E, Perou CM, Ellis M, Winer E, Hudis CA, Berry D, Barry WT (2015) Body mass index, PAM50 subtype, and outcomes in node-positive breast cancer: CALGB 9741 (Alliance). J Natl Cancer Inst. doi:10.1093/jnci/djv179
Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Partridge AH (2015) Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol 33(20):2254–2261. doi:10.1200/JCO.2014.57.1349
Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL (2009) Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 101(14):984–992. doi:10.1093/jnci/djp175
Banegas MP, Li CI (2012) Breast cancer characteristics and outcomes among Hispanic Black and Hispanic White women. Breast Cancer Res Treat 134(3):1297–1304. doi:10.1007/s10549-012-2142-1
Ooi SL, Martinez ME, Li CI (2011) Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat 127(3):729–738. doi:10.1007/s10549-010-1191-6
Parise CA, Bauer KR, Brown MM, Caggiano V (2009) Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J 15(6):593–602. doi:10.1111/j.1524-4741.2009.00822.x
Sweeney C, Bernard PS, Factor RE, Kwan ML, Habel LA, Quesenberry CP, Shakespear K, Weltzien EK, Stijleman IJ, Davis CA, Ebbert MT, Castillo A, Kushi LH, Caan BJ (2014) Intrinsic subtypes from PAM50 gene expression assay in a population-based breast cancer cohort: differences by age, race, and tumor characteristics. Cancer Epidemiol Biomarkers Prev 23(5):714–724. doi:10.1158/1055-9965.EPI-13-1023
O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC (2010) Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina breast cancer study. Clin Cancer Res 16(24):6100–6110. doi:10.1158/1078-0432.CCR-10-1533
Tichy JR, Deal AM, Anders CK, Reeder-Hayes K, Carey LA (2015) Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast Cancer Res Treat 150(3):667–674
Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH (2016) Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2(3):322–329. doi:10.1001/jamaoncol.2015.3856
De Gagliato M, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V, Hortobagyi GN, Chavez-Macgregor M (2014) Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32(8):735–744. doi:10.1200/JCO.2013.49.7693
Gurney H (2002) How to calculate the dose of chemotherapy. Br J Cancer 86(8):1297–1302. doi:10.1038/sj.bjc.6600139
Untch M, Konecny GE, Paepke S, von Minckwitz G (2014) Current and future role of neoadjuvant therapy for breast cancer. Breast 23(5):526–537. doi:10.1016/j.breast.2014.06.004
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804. doi:10.1200/JCO.2011.38.8595
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE (1999) Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17(2):460–469
Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E (2012) Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 48(18):3342–3354. doi:10.1016/j.ejca.2012.05.023
Golshan M, Cirrincione CT, Sikov WM, Berry DA, Jasinski S, Weisberg TF, Somlo G, Hudis C, Winer E, Ollila DW, AfCTi Oncology (2015) Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg 262(3):434–439. doi:10.1097/SLA.0000000000001417 discussion 438–439
von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Kümmel S, Paepke S, Schneeweiss A, Untch M, Zahm DM, Mehta K, Loibl S (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31(29):3623–3630. doi:10.1200/JCO.2012.45.0940
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. doi:10.1016/S0140-6736(13)62422-8
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (2014) Guidance for industry pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. Retrieved from http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf
Griggs JJ, Culakova E, Sorbero ME, van Ryn M, Poniewierski MS, Wolff DA, Crawford J, Dale DC, Lyman GH (2007) Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol 25(3):277–284. doi:10.1200/JCO.2006.08.3063
Griggs JJ, Liu Y, Sorbero ME, Jagielski CH, Maly RC (2014) Adjuvant chemotherapy dosing in low-income women: the impact of Hispanic ethnicity and patient self-efficacy. Breast Cancer Res Treat 144(3):665–672. doi:10.1007/s10549-014-2869-y
Buzdar AU, Suman VJ, Meric-Bernstam F, Leitch AM, Ellis MJ, Boughey JC, Unzeitig G, Royce M, McCall LM, Ewer, Hunt KK, American College of Surgeons Oncology Group i (2013) Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol 14(13):1317–1325. doi:10.1016/S1470-2045(13)70502-3
Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, Ollila DW, Krop IE, Henry NL, Weckstein DJ, Anders CK, Singh B, Hoadley KA, Iglesia M, Cheang MC, Perou CM, Winer EP, Hudis CA (2015) Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. doi:10.1200/JCO.2015.62.1268
Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER, Golshan M, Bellon JR, Collyar D, Hahn OM, Carey LA, Hudis CA, Winer EP (2015) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33(1):13–21. doi:10.1200/JCO.2014.57.0572
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Kuerer HM, Bowling M, Flippo-Morton TS, Byrd DR, Ollila DW, Julian TB, McLaughlin SA, McCall L, Symmans WF, Le-Petross HT, Haffty BG, Buchholz TA, Nelson H, Hunt KK (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310(14):1455–1461. doi:10.1001/jama.2013.278932
Chavez-Macgregor M, Litton J, Chen H, Giordano SH, Hudis CA, Wolff AC, Valero V, Hortobagyi GN, Bondy ML, Gonzalez-Angulo AM (2010) Pathologic complete response in breast cancer patients receiving anthracycline- and taxane-based neoadjuvant chemotherapy: evaluating the effect of race/ethnicity. Cancer 116(17):4168–4177. doi:10.1002/cncr.25296
Killelea BK, Yang VQ, Wang SY, Hayse B, Mougalian S, Horowitz NR, Chagpar AB, Pusztai L, Lannin DR (2015) Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the national cancer data base. J Clin Oncol 33(36):4267–4276. doi:10.1200/JCO.2015.63.7801
Balmanoukian A, Zhang Z, Jeter S, Slater S, Armstrong DK, Emens LA, Fetting JH, Wolff AC, Davidson NE, Jacobs L, Lange J, Tsangaris TN, Zellars R, Gabrielson E, Stearns V (2009) African American women who receive primary anthracycline-and taxane-based chemotherapy for triple-negative breast cancer suffer worse outcomes compared with white women. J Clin Oncol 27(22):e35–e37. doi:10.1200/JCO.2008.21.5509 author reply e38-39
Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW (2003) Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat 81(1):21–31. doi:10.1023/A:1025481505537
Griggs JJ, Culakova E, Sorbero ME, Poniewierski MS, Wolff DA, Crawford J, Dale DC, Lyman GH (2007) Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol 25(18):2522–2527. doi:10.1200/JCO.2006.10.2749
Daly B, Olopade OI (2015) A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin 65(3):221–238. doi:10.3322/caac.21271
Han HS, Reis IM, Zhao W, Kuroi K, Toi M, Suzuki E, Syme R, Chow L, Yip AY, Glück S (2011) Racial differences in acute toxicities of neoadjuvant or adjuvant chemotherapy in patients with early-stage breast cancer. Eur J Cancer 47(17):2537–2545. doi:10.1016/j.ejca.2011.06.027
Litton JK, Gonzalez-Angulo AM, Warneke CL, Buzdar AU, Kau SW, Bondy M, Mahabir S, Hortobagyi GN, Brewster AM (2008) Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol 26(25):4072–4077. doi:10.1200/JCO.2007.14.4527
Chen S, Chen CM, Zhou Y, Zhou RJ, Yu KD, Shao ZM (2012) Obesity or overweight is associated with worse pathological response to neoadjuvant chemotherapy among Chinese women with breast cancer. PLoS One 7(7):e41380. doi:10.1371/journal.pone.0041380
Elsamany S, Alzahrani A, Abozeed WN, Rasmy A, Farooq MU, Elbiomy MA, Rawah E, Alsaleh K, Abdel-Aziz NM (2015) Mammographic breast density: predictive value for pathological response to neoadjuvant chemotherapy in breast cancer patients. Breast 24(5):576–581. doi:10.1016/j.breast.2015.05.007
Bao J, Borja N, Rao M, Huth J, Leitch AM, Rivers A, Wooldridge R, Rao R (2015) Impact of weight change during neoadjuvant chemotherapy on pathologic response in triple-negative breast cancer. Cancer Med 4(4):500–506. doi:10.1002/cam4.388
Kogawa T, Fouad TM, Wei C, Masuda H, Kai K, Fujii T, El-Zein R, Chavez-MacGregor M, Litton JK, Brewster A, Alvarez RH, Hortobagyi GN, Valero V, Theriault R, Ueno NT (2015) Association of body mass index changes during neoadjuvant chemotherapy with pathologic complete response and clinical outcomes in patients with locally advanced breast cancer. J Cancer 6(4):310–318. doi:10.7150/jca.10580
Ligibel JA, Winer EP (2012) Aromatase inhibition in obese women: how much is enough? J Clin Oncol 30(24):2940–2942. doi:10.1200/JCO.2012.43.7244
Ligibel J (2011) Obesity and breast cancer. Oncology 25(11):994–1000
Goodwin PJ, Ennis M, Bahl M, Fantus IG, Pritchard KI, Trudeau ME, Koo J, Hood N (2009) High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat 114(3):517–525. doi:10.1007/s10549-008-0019-0
Irwin ML, Duggan C, Wang CY, Smith AW, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard-Barbash R (2011) Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol 29(1):47–53. doi:10.1200/JCO.2010.28.4752
Lyman GH (2012) Weight-based chemotherapy dosing in obese patients with cancer: back to the future. J Oncol Pract 8(4):e62–e64. doi:10.1200/JOP.2012.000606
Lyman GH, Sparreboom A (2013) Appropriate chemotherapy dosing in obese patients with cancer. Nat Rev Clin Oncol 10(11):664. doi:10.1038/nrclinonc.2013.108-c2
Mougalian SS, Hernandez M, Lei X, Lynch S, Kuerer HM, Symmans WF, Theriault RL, Fornage BD, Hsu L, Buchholz TA, Sahin AA, Hunt KK, Yang WT, Hortobagyi GN, Valero V (2015) Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. doi:10.1001/jamaoncol.2015.4935
Keenan T, Moy B, Mroz EA, Ross K, Niemierko A, Rocco JW, Isakoff S, Ellisen LW, Bardia A (2015) Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J Clin Oncol 2015(2062):2126
Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN, Symmans WF, Ueno NT (2013) Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 19(19):5533–5540. doi:10.1158/1078-0432.CCR-13-0799
Kalata P, Martus P, Zettl H, Rödel C, Hohenberger W, Raab R, Becker H, Liersch T, Wittekind C, Sauer R, Fietkau R, Group GRCS (2009) Differences between clinical trial participants and patients in a population-based registry: the German rectal cancer study vs. the rostock cancer registry. Dis Colon Rectum 52(3):425–437. doi:10.1007/DCR.0b013e318197d13c
Loibl S, Jackisch C, Lederer B, Untch M, Paepke S, Kümmel S, Schneeweiss A, Huober J, Hilfrich J, Hanusch C (2015) Outcome after neoadjuvant chemotherapy in young breast cancer patients: a pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat 152(2):377–387
Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Flippo-Morton T, Hunt KK (2014) Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg 260(4):608–614. doi:10.1097/SLA.0000000000000924 discussion 614-606
Acknowledgments
The authors would like to acknowledge Dr. Olwen Hahn for her contributions to the included studies. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (to the Alliance for Clinical Trials in Oncology NCORP Grant), U10CA180790, U10CA180838, U10CA180858, and U10CA180867. Dr. Warner was supported by NCI Grant K01CA188075. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study sponsors had no role in study design, collection, analysis, and interpretation of data, writing the report, or the decision to submit the report for publication. Dr. Warner had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Warner, E.T., Ballman, K.V., Strand, C. et al. Impact of race, ethnicity, and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer: a pooled analysis of four prospective Alliance clinical trials (A151426). Breast Cancer Res Treat 159, 109–118 (2016). https://doi.org/10.1007/s10549-016-3918-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3918-5