Abstract
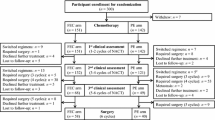
Adding a taxane to anthracycline-based adjuvant chemotherapy prolongs survival in node-positive early breast cancer. However, which is the preferable taxane in a dose-dense regimen remains unknown. We conducted a randomized study to compare the efficacy of dose-dense paclitaxel versus docetaxel following 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) as adjuvant chemotherapy in women with node-positive early breast cancer. Following surgery women with HER2-negative breast cancer and at least one infiltrated axillary lymph node were randomized to receive four cycles of FEC (700/75/700 mg/m2) followed by four cycles of either paclitaxel (175 mg/m2) or docetaxel (75 mg/m2). All cycles were administered every 14 days with G-CSF support. The primary endpoint was disease-free survival (DFS) at 3 years. Between 2004 and 2007, 481 women were randomized to paclitaxel (n = 241) and docetaxel (n = 240). After a median follow-up of 6 years, 51 (21 %) and 48 (20 %) women experienced disease relapse (p = 0.753) and there was no significant difference in DFS between the paclitaxel- and docetaxel-treated groups (3-year DFS 87.4 vs. 88.3 %, respectively; median DFS not reached; p = 0.633). Toxicities were manageable, with grade 2–4 neutropenia in 21 versus 31 % (p = 0.01), thrombocytopenia 0.8 versus 3.4 % (p = 0.06), any grade neurotoxicity 17 versus 7.5 % (p = 0.35) and onycholysis 4.9 versus 12.1 % (p = 0.03) for patients receiving paclitaxel and docetaxel, respectively. There were no toxic deaths. Dose-dense paclitaxel versus docetaxel after FEC as adjuvant chemotherapy results in a similar 3-year DFS rate in women with axillary node-positive early breast cancer. Due to its more favorable toxicity profile, paclitaxel is the taxane of choice in this setting.


Similar content being viewed by others
References
Radcliffe Infirmary CTSU (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM et al (2005) Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23:3686–3696
Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ et al (2003) Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976–983
Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313
Mackey JR, Martin M, Pienkowski T, Rolski J, Guastalla JP et al (2013) Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol 14:72–80
Roche H, Fumoleau P, Spielmann M, Canon JL, Delozier T et al (2006) Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 trial. J Clin Oncol 24:5664–5671
Martin M, Rodriguez-Lescure A, Ruiz A, Alba E, Calvo L et al (2008) Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst 100:805–814
Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER et al (2010) Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 362:2053–2065
Eiermann W, Pienkowski T, Crown J, Sadeghi S, Martin M et al (2011) Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol 29:3877–3884
Swain SM, Tang G, Geyer CE Jr, Rastogi P, Atkins JN et al (2013) Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: the NSABP B-38 trial. J Clin Oncol 31:3197–3204
Saloustros E, Mavroudis D, Georgoulias V (2008) Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother 9:2603–2616
Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins L et al (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542–5551
Seidman AD, Berry D, Cirrincione C, Harris L, Muss H et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 non overexpressors: final results of cancer and leukemia group B protocol 9840. J Clin Oncol 26:1642–1649
Sparano JA, Wang M, Martino S, Jones V, Perez EA et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Bonilla L, Ben-Aharon I, Vidal L, Gafter-Gvili A, Leibovici L et al (2010) Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst 102:1845–1854
Piedbois P, Serin D, Priou F, Laplaige P, Greget S et al (2007) Dose-dense adjuvant chemotherapy in node-positive breast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann Oncol 18:52–57
Trudeau M, Charbonneau F, Gelmon K, Laing K, Latreille J et al (2005) Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol 6:886–898
Polyzos A, Malamos N, Boukovinas I, Adamou A, Ziras N et al (2010) FEC versus sequential docetaxel followed by epirubicin/cyclophosphamide as adjuvant chemotherapy in women with axillary node-positive early breast cancer: a randomized study of the Hellenic Oncology Research Group (HORG). Breast Cancer Res Treat 119:95–104
Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup trial C9741/cancer and leukemia group B trial 9741. J Clin Oncol 21:1431–1439
Jones RL, Walsh G, Ashley S, Chua S, Agarwal R et al (2009) A randomised pilot phase II study of doxorubicin and cyclophosphamide (AC) or epirubicin and cyclophosphamide (EC) given 2 weekly with pegfilgrastim (accelerated) vs 3 weekly (standard) for women with early breast cancer. Br J Cancer 100:305–310
Burnell M, Shepherd K, Gelmon K, Bramwell V, Walley B, et al (2012) A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: results of the final relapse free survival analysis. Cancer Res 72 [24 Suppl 3, abstr P1-13-01]
Budd GT, Barlow WE, Moore HCF, Hobday TJ, Stewart JA, et al (2013) Comparison of two schedules of paclitaxel as adjuvant therapy for breast cancer. J Clin Oncol 31 (suppl; abstr CRA1008)
Acknowledgments
We acknowledge the assistance of Dora Hatzidaki and Vasso Athanasaki in the preparation of this manuscript.
Conflict of interest
The authors have declared no pertinent conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov Identifier: NCT00431080.
Rights and permissions
About this article
Cite this article
Saloustros, E., Malamos, N., Boukovinas, I. et al. Dose-dense paclitaxel versus docetaxel following FEC as adjuvant chemotherapy in axillary node-positive early breast cancer: a multicenter randomized study of the Hellenic Oncology Research Group (HORG). Breast Cancer Res Treat 148, 591–597 (2014). https://doi.org/10.1007/s10549-014-3202-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3202-5