Abstract
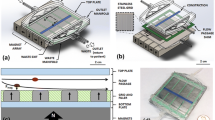
This paper reports on a microfluidic platform to isolate and study avian red blood cells (RBCs) infected to various degrees by the malaria parasite Plasmodium gallinaceum. The experimental findings point to the feasibility of using the morphological changes on the surface of the malaria infected avian RBC (miaRBCs) as biomarkers for diagnosis. A glass substrate with a controlled surface roughness was used as part of a polydimethylsiloxane (PDMS) microfluidic channels. When whole-blood samples were introduced into the channels, the miaRBCs would be preferentially slowed and eventually become immobilized on the roughened surface. The surface lesions and furrow-like structures on the miaRBC surfaces offered a markedly higher probability to interact with the roughened substrate and allowed the cells to become imobilized on the surface. The captured miaRBCs were from blood samples at various degrees of infection at 3.2%, 3.9%, 9.1%, 13.4%, 20.1%, 28%, and 37%. It was observed that the miaRBCs could be selectively captured under a wall shear rate between 2.1 to 3.2 s−1, which was directly proportional to the flow rate through the channels. This capture rate could be improved by increasing the channel length and finer flow control. It was also found that a roughened glass substrate with ten-point-height larger than the depth of surface lesions and furrow-like structures of miaRBCs showed a substantial enhancement on the number of immobilized infected RBCs. These findings indicated that surface morphologies, including surface lesions and furrow-like structures, can serve as an alternative biomarker for malaria diagnosis.












Similar content being viewed by others
References
Y. Adams, C. Freeman, R. Schwartz-Albiez, V. Ferro, C.R. Parish, K.T. Andrews, Antimicrob Agents Ch. 50, 2850 (2006)
M. Antia, T. Herricks, P.K. Rathod, PLoS Pathog. 3, 939 (2007)
B.J. Bain, F.R.A.C.P., F.R.C.Path, New Engl. J. Med. 353, 498 (2005)
L. Bannister, G. Mitchell, Trends Parasitol. 19, 209 (2003)
B.M. Cooke, N. Mohandas, A.F. Cowman, R.L. Coppel, Vet. Parasitol. 132, 273 (2005)
P.J. Freidlin, Avian Pathol. 14, 531 (1985)
P. Gaehtgens, F. Schmidt, G. Will, Pflugers Arch. 390, 278 (1981)
T. Hänscheid, J. Melo-Cristino, B.D. Pinto, Am. J. Trop. Med. Hyg. 64, 290 (2001)
H.W. Hou, A.A.S. Bhagat, A.G.L. Chong, P. Mao, K.S.W. Tan, J. Han, C.T. Lim, Lab Chip 10, 2605 (2010)
P.J. Hung, P.J. Lee, P. Sabounchi, N. Aghdam, R. Lin, L.P. Lee, Lab Chip 5, 44 (2005)
P.J. Lee, P.J. Hung, V.M. Rao, L.P. Lee, Biotechnol. Bioeng. 94, 5 (2006)
E. Nagao, T. Arie, D.W. Dorward, R.M. Fairhurst, J.A. Dvorak, J. Struct. Biol. 162, 460 (2007)
G.B. Nash, B.M. Cooke, K. Marsh, A. Berendt, C. Newbold, J. Stuart, Blood 79, 798 (1992)
J.P. Shelby, J. White, K. Ganesan, P.K. Rathod, D.T. Chiu, PNAS 100, 14618 (2003)
S. Suresh, J. Spatz, J.P. Mills, A. Micoulet, M. Dao, C.T. Lim, M. Beil, T. Seufferlein, Acta Biomater. 5, 15 (2005)
Y. Xia, G.M. Whitesides, Annu. Rev. Mater. Sci. 28, 153 (1998)
L. Xiao, C. Yang, P.S. Patterson, V. Udhayakumar, A.A. Lal, Infect. Immun. 64, 1373 (1996)
Acknowledgments
This work was partially funded by the Defense Advanced Research Projects Agency (DARPA) N/MEMS S&T Fundamentals program under grant no. HR001-06-1-0500 issued to the Micro/nano Fluidics Fundamentals Focus (MF3) Center and the Undergraduate Research Opportunity Program. The authors wish to thank Dr. Anthony A. James for supplying avian blood samples, Dr. Abraham Lee for providing the inverted microscope, and Dr. Jian-Guo Zheng for AFM measurements. Thanks also go to Chih-Jen Kuan for valuable discussions and suggestions. We also acknowledge the assistances from H. Wong, G. Eslamian, J. T. N. Nguyen, S. Ahrar, and K. Cho of the Microbiomechanics Laboratory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
(MPG 692 kb)
(MPG 74 kb)
(MPG 88 kb)
Rights and permissions
About this article
Cite this article
Hsu, YH., Lu, P., Coleman, J.L. et al. A microfluidic platform to isolate avian erythrocytes infected with Plasmodium gallinaceum malaria parasites based on surface morphological changes. Biomed Microdevices 13, 995–1004 (2011). https://doi.org/10.1007/s10544-011-9569-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-011-9569-8