Abstract
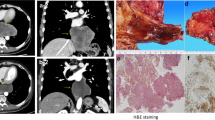
We report a case of esophagectomy after a primary esophageal gastrointestinal stromal tumor (GIST) was preoperatively treated with imatinib mesylate. A 71-year-old woman was diagnosed with an esophageal submucosal tumor by upper gastrointestinal endoscopy at her health checkup. The tumor was located at the lower thoracic esophagus immediately above the esophagogastric junction and measured 4.5 cm in size. It was diagnosed as GIST of the esophagus for reasons of its high susceptibility to imatinib mesylate. Preoperative treatment with imatinib was performed in an attempt to preserve the esophagus. Although the tumor size was decreased by 36% after the 6-month treatment, transhiatal esophagectomy was required for complete resection, and esophageal preservation could not be accomplished.
Similar content being viewed by others
References
Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer 2002;38:S39–S51.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Robert PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–480.
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577–580.
Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708–710.
Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620–625.
Demetri GD, Benjamin R, Blanke CD, Choi H, Corless C, DeMatteo RP, et al. NCCN task force report. Optimal management of patients with gastrointestinal stromal tumor (GIST): expansion and update of NCCN clinical practice guideline. J Natl Comprehensive Cancer Network 2004;2(suppl 1):S1–S26.
Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST consensus conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol 2005;16:566–578.
Al-Salam S, El-Teraifi HA, Taha MS. Could imatinib replace surgery in esophageal gastrointestinal stromal tumor. Saudi Med J 2006;27:1236–1239.
Sakurai N, Yamauchi J, Shibuma H, Ikeda E, Sasou S. A case of recurrent GIST of the esophagus which completely responded to imatinib mesilate. Gan To Kagaku Ryoho 2007;34:237–240.
Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342–4349.
Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol 2000;24:211–222.
Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benkamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626–632.
Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumours: best monitored with FDG PET. Nucl Med Commun 2004;25:433–438.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, H., Kanda, T., Hirota, S. et al. Surgical resection of gastrointestinal stromal tumor of esophagus following preoperative imatinib treatment: a case report. Esophagus 7, 65–69 (2010). https://doi.org/10.1007/s10388-009-0217-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-009-0217-9