Abstract
Background
The expression of programmed death ligand 1 (PD-L1) is considered a predictive biomarker of anti-programmed death 1 (PD-1)/PD-L1 cancer therapies. However, changes in PD-L1 expression of tumor cells during clinical courses have not been fully evaluated. We evaluated changes in PD-L1 expression for non-small cell lung cancer (NSCLC) patients who received anticancer treatments during clinical courses.
Methods
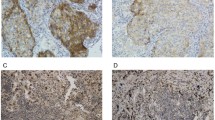
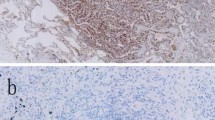
In 76 NSCLC patients, PD-L1 expression was evaluated before and after anticancer treatment by immunohistochemical (IHC) analysis using an anti-PD-L1 antibody. We defined two cut-off points of PD-L1 expression (1 and 50%) and three corresponding IHC groups (A: 0%, B: 1–49%, and C: ≥50%). IHC group B and C were considered to be positive expression, and we defined the difference of IHC group between pre- and post-treatment as ‘major change’ in PD-L1 expression.
Results
Before anticancer treatment, PD-L1 expression was observed in 38/76 (50%) patients, and was significantly less common in patients harboring mutations in the epidermal growth factor receptor gene (EGFR) than in those without (P = 0.039). After anticancer treatment, PD-L1 expression was observed in 36/76 (47%) patients. Major increases in PD-L1 expression were seen in 11 (14%), and major decreases in 18 (24%) patients. Among 13 patients harboring EGFR mutations treated with EGFR tyrosine-kinase inhibitor (EGFR-TKI), five (38%) showed major increases.
Conclusion
Major changes of PD-L1 expression in tumor cells were observed in 38% of NSCLC patients who received anticancer treatments. And, treatments with EGFR-TKI may increase PD-L1 expression in NSCLC patients harboring EGFR mutations.



Similar content being viewed by others
References
Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11(2):121–128
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388
Zhou C, Wu YL, Chen G et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12(8):735–742
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246
Sequist LV, Yang JC, Yamamoto N et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31(27):3327–3334
Wu YL, Zhou C, Hu CP et al (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15(2):213–222
Mok TS, Wu YL, Ahn MJ et al (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376(7):629–640
Solomon BJ, Mok T, Kim DW et al (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371(23):2167–2177
Hida T, Nokihara H, Kondo M et al (2017) Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 390(10089):29–39
Shaw AT, Kim AT, Crino L et al (2017) Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 18(7):874–886
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Borghaei H, Paz-Ares L, Horn L et al (2015) nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639
Brahmer J, Reckamp KL, Baas P et al (2015) nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265
Garassino MC, Cho BC, Kim JH et al (2018) Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 19(4):521–536
Chen YB, Mu CY, Huang JA (2012) Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 98(6):751–755
Velcheti V, Schalper KA, Carvajal DE et al (2014) Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 94(1):107–116
Yang CY, Lin MW, Chang YL et al (2014) Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 50(7):1361–1369
Cooper WA, Tran T, Vilain RE et al (2015) PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 89(2):181–188
Pan ZK, Ye F, Wu X et al (2015) Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis 7(3):462–470
Wang A, Wang HY, Liu Y et al (2015) The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 41(4):450–456
Zhou ZJ, Zhan P, Song Y (2015) PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 4(2):203–208
Yatabe Y, Hida T, Horio Y et al (2006) A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung cancer. J Mol Diagn 8(3):335–341
Kimura H, Kasahara K, Kawaishi M et al (2006) Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin Cancer Res 12(13):3915–3921
Garon EB, Rizvi NA, Hui R et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372(21):2018–2028
Gainor JF, Sequist LV, Shaw AT et al. (2015) Clinical correlation and frequency of programmed death ligand-1 (PD-L1) expression in EGFR-mutant and ALK-rearranged non-small cell lung cancer (NSCLC). ASCO Meeting Abstracts 33(15_suppl):8012
Han JJ, Kim DW, Koh J et al (2016) Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer 17(4):263–270
Meng F, Wang F, Wang L et al (2016) MiR-30a-5p overexpression may overcome EGFR-inhibitor resistance through regulating PI3K/AKT signaling pathway in non-small cell lung cancer cell lines. Front Genet 7:197
Ota K, Azuma K, Kawahara A et al (2015) Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res 21(17):4014–4021
Chen N, Fang W, Zhan J et al (2015) Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol 10(6):910–923
Akbay EA, Koyama S, Carretero J et al (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3(12):1355–1363
Azuma K, Ota K, Kawahara A et al (2014) Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 25(10):1935–1940
D’Incecco A, Andreozzi M, Ludovini V et al (2015) PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 112(1):95–102
Tang Y, Fang W, Zhang Y et al (2015) The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 6(16):14209–14219
Herbst RS, Baas P, Kim DW et al (2015) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Lee CK, Man J, Lord S et al (2017) Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol 12(2):403–407
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230):124–128
Spigel DR, Schrock AB, Fabrizio D et al (2016) Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 34(15_suppl):9017–9017
Madore J, Strbenac D, Vilain R et al (2016) PD-L1 negative status is associated with lower mutation burden, differential expression of immune-related genes, and worse survival in stage III melanoma. Clin Cancer Res 22(15):3915–3923
Katsuya Y, Fujita Y, Horinouchi H et al (2015) Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer 88(2):154–159
Schultheis AM, Scheel AH, Ozretic L et al (2015) PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 51(3):421–426
Gaule P, Smithy JW, Toki M et al (2016) A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol 3(2):256–259
Rimm DL, Han G, Taube JM et al (2017) A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 3(8):1051–1058
Ilie M, Long-Mira E, Bence C et al (2016) Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 27(1):147–153
Kitazono S, Fujiwara Y, Tsuta K et al (2015) Reliability of small biopsy samples compared with resected specimens for the determination of programmed death-ligand 1 expression in non-small-cell lung cancer. Clin Lung Cancer 16(5):385–390
Fehrenbacher L, Spira A, Ballinger M et al (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387(10030):1837–1846
Acknowledgements
We thank all the clinical pathology technicians who supported this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Shota Omori received personal fees from AstraZeneca, Boehriner Ingelheim, Chugai-Pharm, Taiho-Pharm, MSD and Ono-Pharma; Dr. Hirotsugu Kenmotsu received personal fees from AstraZeneca, Chugai-Pharma, Bristol-Myers, Boehriner Ingelheim, Eli Lilly, Kyowa Hakko Kirin, Taiho-Pharma, MSD and Ono-Pharma; Dr. Haruki Kobayashi received personal fees from Eli Lilly, and Taiho-Pharma; Dr. Kazuhisa Nakashima received personal fees from Eli Lilly, Taiho-Pharma, Mochida-Pharma and Ono-Pharma; Dr. Kazushige Wakuda received personal fees from Taiho-Pharma, Boehriner Ingelheim and Ono-Pharma; Dr. Akira Ono received personal fees from Chugai-Pharma, Takeda-Pharma and Taiho-Pharma; Dr. Tateaki Naito received personal fees from Ono-Pharma; Dr. Haruyasu Murakami received personal fees from AstraZeneca, Astellas, Bristol-Myers, Boehriner Ingelheim, Chugai-Pharma, Eli Lilly, Taiho-Pharma, MSD, Pfizer, Novartis and Ono-Pharma; Dr. Masahiro Endo received personal fees from Ono-Pharma; Dr. Toshiaki Takahashi received personal fees from AstraZeneca, Boehriner Ingelheim, Chugai-Pharma, Taiho-Pharma, MSD, Eli Lilly, Pfizer and Ono-Pharma, outside the submitted work. The other authors declare no conflict of interests.
About this article
Cite this article
Omori, S., Kenmotsu, H., Abe, M. et al. Changes in programmed death ligand 1 expression in non-small cell lung cancer patients who received anticancer treatments. Int J Clin Oncol 23, 1052–1059 (2018). https://doi.org/10.1007/s10147-018-1305-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1305-4