Abstract
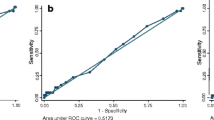
The measurement of antibody levels is a common test for the diagnosis of Streptococcus pneumoniae infection in research. However, the quality of antibody response, reflected by avidity, has not been adequately evaluated. We aimed to evaluate the role of avidity of IgG against eight pneumococcal proteins in etiologic diagnosis. Eight pneumococcal proteins (Ply, CbpA, PspA1 and 2, PcpA, PhtD, StkP-C, and PcsB-N) were used to develop a multiplex bead-based avidity immunoassay. The assay was tested for effects of the chaotropic agent, multiplexing, and repeatability. The developed assay was applied to paired samples from children with or without pneumococcal disease (n = 38 for each group), determined by either serology, polymerase chain reaction (PCR), or blood culture. We found a good correlation between singleplex and multiplex assays, with r ≥ 0.94.The assay was reproducible, with mean inter-assay variation ≤ 9% and intra-assay variation < 6%. Children with pneumococcal disease had lower median avidity indexes in the acute phase of disease for PspA1 and 2 (p = 0.042), PcpA (p = 0.002), PhtD (p = 0.014), and StkP-C (p < 0.001). When the use of IgG avidity as a diagnostic tool for pneumococcal infection was evaluated, the highest discriminative power was found for StkP-C, followed by PcpA (area under the curve [95% confidence interval, CI]: 0.868 [0.759–0.977] and 0.743 [0.607–879], respectively). The developed assay was robust and had no deleterious influence from multiplexing. Children with pneumococcal disease had lower median avidity against five pneumococcal proteins in the acute phase of disease compared to children without disease.






Similar content being viewed by others
References
O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T; Hib and Pneumococcal Global Burden of Disease Study Team (2009) Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902
Fried AJ, Altrich ML, Liu H, Halsey JF, Bonilla FA (2013) Correlation of pneumococcal antibody concentration and avidity with patient clinical and immunologic characteristics. J Clin Immunol 33:847–856
Ekström N, Ahman H, Palmu A, Grönholm S, Kilpi T, Käyhty H; FinOM Study Group (2013) Concentration and high avidity of pneumococcal antibodies persist at least 4 years after immunization with pneumococcal conjugate vaccine in infancy. Clin Vaccine Immunol 20:1034–1040
Oishi T, Ishiwada N, Matsubara K, Nishi J, Chang B, Tamura K, Akeda Y, Ihara T, Nahm MH, Oishi K; Japanese IPD Study Group (2013) Low opsonic activity to the infecting serotype in pediatric patients with invasive pneumococcal disease. Vaccine 31:845–849
Principi N, Esposito S (2011) Universal protein vaccines against Neisseria meningitidis serogroup B, Streptococcus pneumoniae and influenza. Hum Vaccin 7:905–912
van der Poll T, Opal SM (2009) Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374:1543–1556
Tai SS (2006) Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit Rev Microbiol 32:139–153
Brooks-Walter A, Briles DE, Hollingshead SK (1999) The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect Immun 67:6533–6542
Briles DE, Hollingshead S, Brooks-Walter A, Nabors GS, Ferguson L, Schilling M, Gravenstein S, Braun P, King J, Swift A (2000) The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine 18:1707–1711
Croney CM, Coats MT, Nahm MH, Briles DE, Crain MJ (2012) PspA family distribution, unlike capsular serotype, remains unaltered following introduction of the heptavalent pneumococcal conjugate vaccine. Clin Vaccine Immunol 19:891–896
Khan MN, Sharma SK, Filkins LM, Pichichero ME (2012) PcpA of Streptococcus pneumoniae mediates adherence to nasopharyngeal and lung epithelial cells and elicits functional antibodies in humans. Microbes Infect 14:1102–1110
Khan MN, Pichichero ME (2012) Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine 30:2900–2907
Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah YA, Barren P, Lathigra R, Langermann S, Koenig S, Johnson S (2001) Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun 69:949–958
Giefing C, Meinke AL, Hanner M, Henics T, Bui MD, Gelbmann D, Lundberg U, Senn BM, Schunn M, Habel A, Henriques-Normark B, Ortqvist A, Kalin M, von Gabain A, Nagy E (2008) Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med 205:117–131
Giefing-Kröll C, Jelencsics KE, Reipert S, Nagy E (2011) Absence of pneumococcal PcsB is associated with overexpression of LysM domain-containing proteins. Microbiology 157:1897–1909
Giefing C, Jelencsics KE, Gelbmann D, Senn BM, Nagy E (2010) The pneumococcal eukaryotic-type serine/threonine protein kinase StkP co-localizes with the cell division apparatus and interacts with FtsZ in vitro. Microbiology 156:1697–1707
Chen A, Mann B, Gao G, Heath R, King J, Maissoneuve J, Alderson M, Tate A, Hollingshead SK, Tweten RK, Briles DE, Tuomanen EI, Paton JC (2015) Multivalent pneumococcal protein vaccines comprising pneumolysoid with epitopes/fragments of CbpA and/or PspA elicit strong and broad protection. Clin Vaccine Immunol 22:1079–1089
Olafsdottir TA, Lingnau K, Nagy E, Jonsdottir I (2012) Novel protein-based pneumococcal vaccines administered with the Th1-promoting adjuvant IC31 induce protective immunity against pneumococcal disease in neonatal mice. Infect Immun 80:461–468
Brooks WA, Chang LJ, Sheng X, Hopfer R; PPR02 Study Team (2015) Safety and immunogenicity of a trivalent recombinant PcpA, PhtD, and PlyD1 pneumococcal protein vaccine in adults, toddlers, and infants: a phase I randomized controlled study. Vaccine 33:4610–4617
Andrade DC, Borges IC, Ivaska L, Peltola V, Meinke A, Barral A, Käyhty H, Ruuskanen O, Nascimento-Carvalho CM (2016) Serological diagnosis of pneumococcal infection in children with pneumonia using protein antigens: a study of cut-offs with positive and negative controls. J Immunol Methods 433:31–37
Andrade DC, Borges IC, Laitinen H, Ekström N, Adrian PV, Meinke A, Barral A, Nascimento-Carvalho CM, Käyhty H (2014) A fluorescent multiplexed bead-based immunoassay (FMIA) for quantitation of IgG against Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis protein antigens. J Immunol Methods 405:130–143
Jiménez-Munguía I, van Wamel WJ, Olaya-Abril A, García-Cabrera E, Rodríguez-Ortega MJ, Obando I (2015) Proteomics-driven design of a multiplex bead-based platform to assess natural IgG antibodies to pneumococcal protein antigens in children. J Proteome 126:228–233
Meriluoto M, Hedman L, Tanner L, Simell V, Mäkinen M, Simell S, Mykkänen J, Korpelainen J, Ruuskanen O, Ilonen J, Knip M, Simell O, Hedman K, Söderlund-Venermo M (2012) Association of human bocavirus 1 infection with respiratory disease in childhood follow-up study, Finland. Emerg Infect Dis 18:264–271
Chen T, Tanner L, Simell V, Hedman L, Mäkinen M, Sadeghi M, Veijola R, Hyöty H, Ilonen J, Knip M, Toppari J, Simell O, Söderlund-Venermo M, Hedman K (2014) Diagnostic methods for and clinical pictures of polyomavirus primary infections in children, Finland. Emerg Infect Dis 20:689–692
Ota MO, Oluwalana C, Howie SR, Gomez M, Ogunniyi AD, Mendy-Gomez AL, Owolabi O, Mureithi MW, Townend J, Secka O, Antonio M, Sutherland JS, Adegbola RA (2011) Antibody and T-cell responses during acute and convalescent stages of invasive pneumococcal disease. Int J Infect Dis 15:e282–e288
Posfay-Barbe KM, Galetto-Lacour A, Grillet S, Ochs MM, Brookes RH, Kraehenbuhl JD, Cevey-Macherel M, Gehri M, Gervaix A, Siegrist CA (2011) Immunity to pneumococcal surface proteins in children with community-acquired pneumonia: a distinct pattern of responses to pneumococcal choline-binding protein A. Clin Microbiol Infect 17:1232–1238
Seiberling M, Bologa M, Brookes R, Ochs M, Go K, Neveu D, Kamtchoua T, Lashley P, Yuan T, Gurunathan S (2012) Safety and immunogenicity of a pneumococcal histidine triad protein D vaccine candidate in adults. Vaccine 30:7455–7460
Ogunniyi AD, Woodrow MC, Poolman JT, Paton JC (2001) Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect Immun 69:5997–6003
Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, Abouseada N, Oldfield NJ, Self T, Ala’Aldeen DA, Tuomanen EI (2009) Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest 119:1638–1646
Borges IC, Andrade DC, Cardoso MR, Toppari J, Vähä-Mäkilä M, Ilonen J, Knip M, Hyöty H, Veijola R, Simell O, Jartti T, Käyhty H, Ruuskanen O, Nascimento-Carvalho CM (2016) Natural development of antibodies against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis protein antigens during the first 13 years of life. Clin Vaccine Immunol 23:878–883
Pullen GR, Fitzgerald MG, Hosking CS (1986) Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods 86:83–87
Ekström N, Väkeväinen M, Verho J, Kilpi T, Käyhty H (2007) Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect Immun 75:1794–1800
Anttila M, Voutilainen M, Jäntti V, Eskola J, Käyhty H (1999) Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin Exp Immunol 118:402–407
Schlesinger Y, Granoff DM (1992) Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA 267:1489–1494
Usinger WR, Lucas AH (1999) Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun 67:2366–2370
Musher DM, Phan HM, Watson DA, Baughn RE (2000) Antibody to capsular polysaccharide of Streptococcus pneumoniae at the time of hospital admission for pneumococcal pneumonia. J Infect Dis 182:158–167
Rapola S, Kilpi T, Lahdenkari M, Mäkelä PH, Käyhty H (2001) Antibody response to the pneumococcal proteins pneumococcal surface adhesin A and pneumolysin in children with acute otitis media. Pediatr Infect Dis J 20:482–487
Andrade DC, Borges IC, Adrian PV, Meinke A, Barral A, Ruuskanen O, Käyhty H, Nascimento-Carvalho CM (2016) Effect of pneumococcal conjugate vaccine on the natural antibodies and antibody responses against protein antigens from Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in children with community-acquired pneumonia. Pediatr Infect Dis J 35:683–689
Salt P, Banner C, Oh S, Yu LM, Lewis S, Pan D, Griffiths D, Ferry B, Pollard A (2007) Social mixing with other children during infancy enhances antibody response to a pneumococcal conjugate vaccine in early childhood. Clin Vaccine Immunol 14:593–599
Acknowledgements
We thank Sanofi Pasteur (Lyon, France) for supplying PcpA and PhtD; Prof. Elaine Tuomanen at St. Jude Children’s Research Hospital (Memphis, TN, USA) for supplying Ply, CbpA, and PspA1; Profs. Susan Hollingshead, David Briles, and Pat Coan at University of Alabama (Birmingham, AL, USA) for supplying PspA2; and Valneva Austria GmbH (Vienna, Austria) for supplying StkP-C and PcsB-N.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by: Bahia State Agency for Research Funding (FAPESB), Brazil; Brazilian Council for Scientific and Technological Development (CNPq), Brazil; Turku University Hospital Research Foundation, Finland; Rauno and Anne Puolimatka Foundation, Finland; Sohlberg Foundation, Finland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The use of the samples was approved by the Ethics Committee of the Federal University of Bahia in Brazil, the Ethics Committee of the National Institute for Health and Welfare in Finland (formerly National Public Health Institute), and the Ethics Committee of Satakunta Central Hospital, Pori, Finland.
Informed consent
Written informed consent was obtained from legal guardians before recruitment.
Rights and permissions
About this article
Cite this article
Andrade, D.C., Borges, I.C., Ekström, N. et al. Determination of avidity of IgG against protein antigens from Streptococcus pneumoniae: assay development and preliminary application in clinical settings. Eur J Clin Microbiol Infect Dis 37, 77–89 (2018). https://doi.org/10.1007/s10096-017-3103-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-3103-8