Abstract
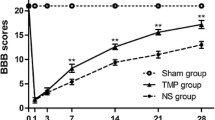
The present study focused on the biologic effects of tempol on anti-inflammatory and nitric oxide generation in contusion spinal cord injury (SCI). The animal model of SCI was induced by dropping a 10-g rod (2.0 mm in diameter) at a height of 25 mm. Tempol was injected intraperitoneally a dose of 100 mg/kg at 15 min before SCI. Controls was injected with saline. The contused spinal segments were removed according to time courses, and the expression level of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) was analyzed along with the size of irreversibly damaged region. After SCI, the relative amounts of COX-2 and iNOS mRNA were peaked at 8 h after post-injury, and then decreased up to 7 days post-injury, and normal level at 14 days. Expression of COX-2 protein was peaked at 8 h post-injury. With the tempol pre-treatment, the immunoreactivity of COX-2 and nitrotyrosine in paraffin-embedded tissue slices was profoundly decreased. The irreversibly damaged area of the spinal cord was peaked at 3 days after SCI. With tempol pre-treatment, the irreversibly damaged area shows a statistically significant decrease at 3 days after SCI. These evidences indicate that tempol pre-treatment reduces irreversibly damaged area on the contusion SCI in rat. The mechanisms of biologic reactions of tempol might be related to the decreased expressions of COX-2 and iNOS in spinal cord cells, neurons and glia. It is expected that the tempol effect on the SCI is not only antioxidant activity but also anti-inflammatory reaction.






Similar content being viewed by others
References
Thiemermann C (2003) Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med 31:S76–S84
Amar AP, Levy ML (1999) Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery 44:1027–1039
Hahn SM, Sullivan FJ, Deluca AM, Bacher JD, Liebmann J, Krishna MC, Coffin D, Mitchell JB (1999) Hemodynamic effect of the nitroxide superoxide dismutase mimics. Free Radic Biol Med 27:529–535
Mitchell JB, Samuni A, Krishna MC, DeGraff WG, Ahn MS, Samuni U, Russo A (1990) Biologically active metal-independent superoxide dismutase mimics. Biochemistry 29:2802–2807
Rak R, Chao DL, Pluta RM, Mitchell JB, Oldfield EH, Watson JC (2000) Neuroprotection by the stable nitroxide tempol during reperfusion in a rat model of transient focal ischemia. J Neurosurg 92:646–651
Deng Y, Singh IN, Carrico KM, Hall ED (2008) Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood F Metab 28:1114–1126
Hillard VH, Peng H, Zhang Y, Das K, Murali R, Etlinger JD, Zeman RJ (2004) Tempol, a nitroxide antioxidant, improves locomotor and histological outcomes after spinal cord contusion in rats. J Neurotrauma 21:1405–1414
Patel SP, Sullivan PG, Pandya JD, Rabchevsky AG (2009) Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol of the nitroxide antioxidant, tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J Neurosci Res 87:130–140
Xiong YQ, Hall ED (2009) Pharmacological evidence for a role of peroxynitrite in the pathophysiology of spinal cord injury. Exp Neurol 216:105–114
Sledzinski Z, Wozniak M, Antosiewicz J, Lezoche E, Familiari M, Bertoli E, Greci L, Brunelli A, Mazera N, Wajda Z (1995) Protective effect of 4-hydroxy-TEMPO, a low molecular weight superoxide dismutase mimic, on free radical toxicity in experimental pancreatitis. Int J Pancreatol 18:153–160
Cuzzocrea S, McDonald MC, Mota-Filipe H, Costantino G, Mazzon E, Santagati S, Caputi AP, Thiemermann C (2000) Effects of tempol, a membrane-permeable radical scavenger, in a rodent model of carrageenan-induced pleurisy. Eur J Pharmacol 390:209–222
Cuzzocrea S, McDonald MC, Mota-Filipe H, Mazzon E, Costantino G, Britti D, Mazzullo G, Caputi AP, Thiemermann C (2000) Beneficial effects of tempol, a membrane-permeable radical scavenger, in a rodent model of collagen-induced arthritis. Arthr Rheum 43:320–328
Cuzzocrea S, McDonald MC, Mazzon E, Dugo L, Lepore V, Fonti MT, Ciccolo A, Terranova ML, Caputi AP, Thiemermann C (2000) Tempol, a membrane-permeable radical scavenger, reduces dinitrobenzene sulfonic acid-induced colitis. Eur J Pharmacol 406:127–137
Xu J, Kim GM, Chen S, Yan P, Ahmed SH, Ku G, Beckman JS, Xu XM, Hsu CY (2001) iNOS and nitrotyrosine expression after spinal cord injury. J Neurotrauma 18:523–532
Dietrich WD, Chatzipanteli K, Vitarbo E, Wada K, Kinoshita K (2004) The role of inflammatory processes in the pathophysiology and treatment of brain and spinal cord trauma. Acta Neurochir Suppl 89:69–74
Kwak EK, Kim JW, Kang KS, Lee YH, Quan HH, Park TI, Park JY, Sohn YK (2005) The role of inducible nitric oxide synthase following spinal cord injury in rat. J Korean Med Sci 20:663–669
Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F (2002) Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthr Cartil 10:792–798
Farooqui AA, Litsky ML, Farooqui T, Horrocks LA (1999) Inhibitors of intracellular phospholipase A2 activity: their neurochemical effects and therapeutical importance for neurological disorders. Brain Res Bull 49:139–153
Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF (1993) Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neurons 11:371–386
Nogawa S, Zhang F, Ross ME, Iadecola C (1997) Cyclooxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci 17:2746–2755
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408
Schultke E, Kendall E, Kamencic H, Ghong Z, Griebel RW, Juurlink BHJ (2003) Quercetin promotes functional recovery following acute spinal cord injury. J Neurotrauma 20:583–591
Hall ED (2001) Pharmacological treatment of acute spinal cord injury: how do we build on past success? J Spinal Cord Med 24:142–146
Nakajima Y, Osuka K, Seki Y, Gupta RC, Hara M, Takayasu M, Wakabayashi T (2010) Taurine reduces inflammatory responses after spinal cord injury. J Neurotrauma 27:403–410
Gomez-Pinilla PJ, Camello PJ, Tresguerres JAF, Pozo MJ (2010) Tempol protects the gallbladder against ischemia/reperfusion. J Physiol Biochem 66:161–172
Adachi K, Yimin Y, Satake K, Matsuyama Y, Ishiguro N, Sawada M, Hirata Y, Kiuchi K (2005) Localization of cyclooxygenase-2 induced following traumatic spinal cord injury. Neurosci Res 51:73–80
Resnick DK, Graham SH, Dixon CE, Marion DW (1998) Role of cyclooxygenase 2 in acute spinal cord injury. J Neurotrauma 15:1005–1013
Tonai T, Taketani Y, Ueda N, Nishisho T, Ohmoto Y, Sakata Y, Muraguchi M, Wada K, Yamamoto S (1999) Possible involvement of interleukin-1 in cyclooxygenase-2 induction after spinal cord injury in rats. J Neurochem 72:302–309
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quan, HH., Kang, KS., Sohn, YK. et al. Tempol reduces injury area in rat model of spinal cord contusion injury through suppression of iNOS and COX-2 expression. Neurol Sci 34, 1621–1628 (2013). https://doi.org/10.1007/s10072-013-1295-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-013-1295-y