Abstract
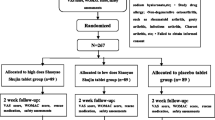
The objective of this study was to evaluate the efficacy and safety of diacerein in early, symptomatic knee osteoarthritis in Indian population. Sixty-four patients of knee osteoarthritis fulfilling American College of Rheumatology Criteria were randomized to receive either diacerein or placebo for 8 weeks, followed by 4 weeks “treatment-free” follow-up in this single-blind, parallel group, post-marketing trial. Primary efficacy variable was visual analogue scale (VAS) assessment of pain on movement; secondary efficacy variables included Western Ontario and Mc Master Universities Osteoarthritis Index (WOMAC) subscores for stiffness and physical function, rescue medication use and physician's clinical global impression (CGI). Compared to placebo, diacerein showed highly significant (p < 0.01) reductions in VAS pain scores, significant (p < 0.05) reductions in WOMAC physical function scores, significantly lower requirement for rescue medication, and significantly better CGI grades. Incidence of adverse events were significantly (p < 0.01) higher in diacerein arm with urine discoloration and soft stool being the most common ones. However, most events were of mild to moderate intensity. In Indian patients with knee osteoarthritis, diacerein effectively reduces pain and improves physical function, and despite frequent adverse events, overall tolerability seemed to be good.
Similar content being viewed by others
References
Sarzi-Puttini P, Cimmino MA, Scarpa R et al (2005) Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum 35:1–10
Wolfe MM, Lichtenstein DR, Singh G (1999) Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 340:1888–1899
Brater DC (2002) Anti-inflammatory agents and renal function. Semin Arthritis Rheum 32:33–42
Silverstein FE, Faich G, Goldstein JL et al (2000) Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-Term Arthritis Safety Study. JAMA 284:1247–1255
Pelletier JP, Martel-Pelletier J (2007) DMOAD developments: present and future. Bull NYU Hosp Jt Dis 65:242–248
Verbruggen G (2006) Chondroprotective drugs in degenerative joint diseases. Rheumatology 45:129–138
Martel Pelletier J, Mineau F, Jolicoeur FC et al (1998) In vitro effects of diacerhein and rhein on IL-1β and TNF-α systems in human osteoarthritic synovium & chondrocytes. J Rheumatol 25:753–762
Moore AR, Greenslade KT, Alan CA et al (1998) Effects of diacerhein on granuloma induced cartilage breakdown in the mouse. Osteoarthr Cartil 6:19–23
Spencer CM, Wilde MI (1997) Diacerein. Drugs 53:98–108
Pelletier JP, Mineau F, Fernandes JC et al (1998) Diacerhein & rhein reduce the IL-1β stimulated inducible nitric oxide synthesis level & activity while stimulating cyclooxygenase-2 synthesis in human osteoarthritic chondrocytes. J Rheumatol 25:2417–2424
Yaron M, Shirazi I, Yaron I (1999) Anti-interleukin-1 effects of diacerein and rhein in human osteoarthritic synovial tissue and cartilage cultures. Osteoarthr Cartil 7:272–280
Mian M, Benetti D, Rosini S et al (1989) Rhein reduces proteoglycan loss during the autolytic breakdown of cultured cartilage. Int J Tissue React 11:117–122
Smith GN Jr, Myers SL, Brandt KD et al (1999) Diacerhein treatment reduces the severity of osteoarthritis in the canine cruciate-deficiency model osteoarthritis. Arthritis Rheum 42:545–554
Dougados M, Nguyen M, Berdah L et al (2001) Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH (Evaluation of Chondroprotective Effect of Diacerein in Osteoarthritis of Hip), a three-year, placebo-controlled trial. Arthritis Rheum 44:2539–2547
Pelletier JP, Yaron M, Haraoui B et al (2000) Efficacy and safety of diacerein in osteoarthritis of the knee: a double-blind placebo-controlled trial. Arthritis Rheum 43:2339–2348
Nguyen M, Dougados M, Berdah L et al (1994) Diacerhein in the treatment of osteoarthritis of the hip. Arthritis Rheum 37:529–536
Fagnani F, Bouvenot G, Valat JP et al (1998) Medico-economic analysis of diacerein with or without standard therapy in the treatment of osteoarthritis. Pharmacoeconomics 13:135–146
Fidelix TS, Soares BG, Trevisani VF(2006) Diacerein for osteoarthritis. Cochrane Database Syst Rev (1):CD005117
Rintelen B, Neumann K, Leeb BF (2006) A metaanalysis of controlled clinical studies with diacerein in the treatment of osteoarthritis. Arch Intern Med 166:1899–1906
Sharma A, Rathod R, Baliga VP (2008) An open prospective study on postmarketing evaluation of the efficacy and tolerability of diacerein in osteoarthritis of the knee. J Indian Med Assoc 106:54–56
World Medical Association Declaration of Helsinki (2000) Ethical principles for medical research involving human subjects. Version amended at the 52nd World Medical Association General Assembly, Edinburgh, Scotland, Oct 2000
Altman R, Asch E, Bloch D et al (1986) Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of knee. Arthritis Rheum 29:1039–1049
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthritis. Ann Rheum Dis 16:494–502
Bellamy N, Buchanan WW, Goldsmith CH et al (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Acknowledgements
The study drug (diacerein), identical-looking placebo capsules, and rescue medication (paracetamol tablets) were provided as complementary samples by Macleods Pharmaceuticals Ltd., Mumbai.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brahmachari, B., Chatterjee, S. & Ghosh, A. Efficacy and safety of diacerein in early knee osteoarthritis: a randomized placebo-controlled trial. Clin Rheumatol 28, 1193–1198 (2009). https://doi.org/10.1007/s10067-009-1225-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-009-1225-9