Abstract
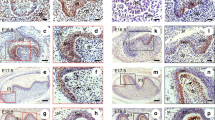
Activating transcription factor 2 (ATF-2/CRE-BP1; cAMP-responsive element binding protein 1) is a member of nuclear transcription factor activator protein-1 (AP-1) family. AP-1 regulates cellular processes including growth, proliferation, differentiation and apoptosis. However, biological relationship of cellular process to each member of the AP-1 family is not clear yet. The objective of the present study was to compare the ATF-2 immunoreactivity in the post-mitotic and terminally differentiated odontoblasts and in the pulpal fibroblasts which can be divided by mitosis when required. Fibroblasts at various stages of differentiation co-exist in the human dental pulp. ATF-2 was investigated immunohistochemically in 20 permanent human teeth. According to the findings obtained, the mean percentage of ATF-2 positive cells was 68.5 ± 19.2 % in the odontoblasts and 22.8 ± 13.7 % in the pulpal fibroblasts. The comparison of ATF-2 positivity revealed a statistically significant difference between odontoblasts and pulpal fibroblasts. These findings have suggested that ATF-2 is more associated with cell survival rather than cell proliferation, and revealed much of effectiveness in maintaining terminal differentiation than the various differentiation stages of the cells.


Similar content being viewed by others
References
Foletta VC (1996) Transcription factor AP-1, and the role of Fra-2. Immunol Cell Biol 74:121–133
Wang L, Payton R, Dai W, Lu L (2011) Hyperosmotic stress-induced ATF-2 activation through Polo-like kinase 3 in human corneal epithelial cells. J Biol Chem 286:1951–1958
Ameyar M, Wisniewska M, Weitzman JB (2003) A role for AP-1 in apoptosis: the case for and against. Biochimie 85:747–752
Fleischmann A, Hafezi F, Elliott C, Reme EC, Rüther U, Wagner FE (2000) Fra-1 replaces c-Fos-dependent functions in mice. Genes Dev 14:2695–2700
Karin M, Liu Z, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9:240–246
Hess J, Angel P, Schorpp-Kistner M (2004) AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117:5965–5973
Milde-Langosch K, Röder H, Andritzky B, Aslan B, Hemminger G, Brinkmann A, Bamberger CM, Löning T, Bamberger AM (2004) The role of the AP-1 transcription factors c-Fos, FosB, Fra-1 and Fra-2 in the invasion process of mammary carcinomas. Breast Cancer Res Treat 86:139–152
Yi JH, Park SW, Kapadia R, Vemuganti R (2007) Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem Int 50:1014–1027
Angel P, Szabowski A (2002) Function of AP-1 target genes in mesenchymal-epithelial cross-talk in skin. Biochem Pharmacol 64:949–956
Kundu JK, Surh YJ (2004) Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat Res 555:65–80
Bolat I, Keklikoglu N (2010) Immunoreactivity of ATF-2 and Fra-2 in human dental follicle. Folia Histochem Cytobiol 48:197–201
Jochum W, Passegue E, Wagner EF (2001) AP-1 in mouse development and tumorigenesis. Oncogene 20:2401–2412
Keklikoglu N (2004) The localization of Fos B, a member of transcription factor AP-1 family, in rat odontoblasts and pulpal undifferentiated ectomesenchymal cells. Folia Histochem Cytobiol 42:191–193
Shirsat NV, Shaikh SA (2003) Overexpression of the immediate early gene fra-1 inhibits proliferation, induces apoptosis, and reduces tumourigenicity of c6 glioma cells. Exp Cell Res 291:91–100
Seong KH, Maekawa T, Ishii S (2012) Inheritance and memory of stress-induced epigenome change: roles played by the ATF-2 family of transcription factors. Genes Cells 17:249–263
Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V (2008) The role of ATF-2 in oncogenesis. Bioessays 30:314–327
Maekawa T, Jin W, Ishii S (2010) The role of ATF-2 family transcription factors in adipocyte differentiation: antiobesity effects of p38 inhibitors. Mol Cell Biol 30:613–625
Bhoumik A, Lopez-Bergami P, Ronai Z (2007) ATF2 on the double—activating transcription factor and DNA damage response protein. Pigment Cell Res 20:498–506
Ouwens DM, de Ruiter ND, van der Zon GC, Carter AP, Schouten J, van der Burgt C, Kooistra K, Bos JL, Maassen JA, van Dam H (2002) Growth factors can activate ATF2 via a two step mechanism: phosphorylation of Thr71 through the Ras-MEK-ERK pathway and of Thr69 through RalGDS-Src-p38. EMBO J 21:3782–3793
Persengiev SP, Green MR (2003) The role of ATF/CREB family members in cell growth, survival and apoptosis. Apoptosis 8:225–228
Bhoumik A, Ronai Z (2008) ATF2: a transcription factor that elicits oncogenic or tumor suppressor activities. Cell Cycle 7:2341–2345
Torneck CD (1994) Dentin Pulp Complex. In: Ten Cate AR (ed) Oral histology development, structure and function, 4th edn. Mosby, St. Louis, pp 169–217
Chiego DJ Jr (2002) Histology of the Pulp. In: Avery JK (ed) Oral development and Histology, 3rd edn. Thieme, New York, pp 190–212
Ruch JV, Lesot H, Bègue-Kirn C (1995) Odontoblast differentiation. Int J Dev Biol 39:51–68
Pashley DH, Walton RE, Slavkin HC (2002) Histology and physiology of the dental pulp. In: Ingle JI, Bakland LK (eds) Endodontics, 5th edn. BC Decker Inc, Hamilton, pp 25–61
Lin LM, Rosenberg PA (2011) Repair and regeneration in endodontics. Int Endod J 44:889–906
Matsushita K, Motani R, Sakuta T, Yamaguchi N, Koga T, Matsuo K, Nagaoka S, Abeyama K, Maruyama I, Torii M (2000) The role of vascular endothelial growth factor in human dental pulp cells: induction of chemotaxis, proliferation, and differentiation and activation of the AP-1-dependent signaling pathway. J Dent Res 79:1596–1603
Nishikawa S (2004) Transient increase in anti-p-ATF2 immunoreactivity in the late secretion ameloblasts apical to the transition zone of rat incisors. Anat Sci Int 79:87–94
Lau E, Kluger H, Varsano T, Lee K, Scheffler I, Rimm DL, Ideker T, Ronai ZA (2012) PKCε promotes oncogenic functions of ATF2 in the nucleus while blocking its apoptotic function at mitochondria. Cell 148:543–555
Lau E, Ronai ZA (2012) ATF2—at the crossroad of nuclear and cytosolic functions. J Cell Sci 125:2815–2824
Liu H, Deng X, Shyu YJ, Li JJ, Taparowsky EJ, Hu CD (2006) Mutual regulation of c-Jun and ATF2 by transcriptional activation and subcellular localization. EMBO J 25:1058–1069
Roivainen A, Söderström KO, Pirilä L, Aro H, Kortekangas P, Merilahti-Palo R, Yli-Jama T, Toivanen A, Toivanen P (1996) Oncoprotein expression in human synovial tissue: an immunohistochemical study of different types of arthritis. Br J Rheumatol 35:933–942
Keklikoglu N, Akinci S (2013) Comparison of three different techniques for histological tooth preparation. Folia Histochem Cytobiol 51:286–291
Keklikoglu N (2008) c-Jun, Fra-2, and ATF-2 immunoreactivity in the jejunal tissues of the healthy rat. Dig Dis Sci 53:2680–2686
Acknowledgments
The authors wish to thank Dr. Humeyra Kocaelli (Istanbul University Faculty of Dentistry Department of Oral Surgery and Medicine, Istanbul, Turkey) for her valuable contribution in providing the necessary materials for this research, and Biologist Ilker Bolat (Istanbul University Faculty of Dentistry Department of Histology and Embryology, Istanbul, Turkey) for his assistance during the experimental work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keklikoglu, N., Akinci, S. ATF-2 immunoreactivity in post-mitotic and terminally differentiated human odontoblasts. Med Mol Morphol 48, 164–168 (2015). https://doi.org/10.1007/s00795-014-0092-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-014-0092-x