Abstract
Background
Tenascin-C (TN-C) is expressed in the cartilage of osteoarthritis (OA). We examined whether TN-C was involved in cartilage repair of the diseased joints. Human articular cartilage samples were obtained from patients with OA and those with normal joints.
Methods
Immunohistochemistry testing of TN-C, chondroitin sulfate (CS), and proliferating cell nuclear antigen (PCNA) was performed. Chondrocytes were isolated from human cartilage and cultured. After treatment with TN-C, chondrocyte proliferation s was analyzed by bromodeoxyuridine (BrdU) incorporation assay using an enzyme-linked immunosorbent assay kit. Glycosaminoglycan content was determined by dimethylmethylene blue (DMMB) assay. The mRNA expression of aggrecan was also analyzed, by quantitative real-time polymerase chain reaction (PCR).
Results
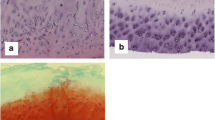
In osteoarthritic cartilage, increased TN-C staining was observed with the degeneration of articular cartilage in comparison with normal cartilage. TN-C staining was shown in the cartilage surface overlying CS-positive areas. In addition, the expression of PCNA in the positive areas for TN-C was significantly higher than that in the negative areas. Treatment of human articular chondrocytes with 10 μg/ml TN-C accelerated chondrocyte proliferation, increased the proteoglycan amount in culture, and increased the expression of aggrecan mRNA.
Conclusions
Our findings indicate that the distribution of TN-C is related to CS production and chondrocyte proliferation in osteoarthritic cartilage and that TN-C has effects on DNA synthesis, proteoglycan content, and aggrecan mRNA expression in vitro. TN-C may be responsible for repair in human osteoarthritic cartilage.
Similar content being viewed by others
References
Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev 2006;58:150–167.
Jenniskens YM, Koevoet W, de Bart AC, Weinans H, Verhaar JA, Jahr H, et al. Biochemical and functional modulation of the cartilage collagen network by IGF1, TGFbeta2 and FGF2. Osteoarthritis Cartilage 2006;14:1136–1146.
Chiquet-Ehrismann R. Tenascins, a growing family of extracellular matrix proteins. Experientia 1995;51:853–862.
Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 1986;47:131–139.
Yoshida T, Matsumoto E, Hanamura N, Kalembeyi I, Katsuta K, Ishihara A, et al. Co-expression of tenascin and fibronectin in epithelial and stromal cells of benign lesions and ductal carcinomas in the human breast. J Pathol 1997;182:421–428.
Yoshida T, Yoshimura E, Numata H, Sakakura Y, Sakakura T. Involvement of tenascin-C in proliferation and migration of laryngeal carcinoma cells. Virchows Arch 1999;435:496–500.
Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, et al. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol 2005;167:71–80.
Mackie EJ, Murphy LI. The role of Tenascin-C and related glycoproteins in early chondrogenesis. Microsc Res Tech 1998;43:102–110.
Pfander D, Heinz N, Rothe P, Carl HD, Swoboda B. Tenascin and aggrecan expression by articular chondrocytes is influenced by interleukin 1β: a possible explanation for the changes in matrix synthesis during osteoarthritis. Ann Rheum Dis 2004;63:240–244.
Chevalier X, Groult N, Larget-Piet B, Zardi L, Hornebeck W. Tenascin distribution in articular cartilage from normal subjects and from patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 1994;37:1013–1022.
Schmidt-Rohlfing B, Gavenis K, Kippels M, Schneider U. New potential markers for cartilage degradation of the knee joint. Scand J Rheumatol 2002;31:151–157.
Hasegawa M, Hirata H, Sudo A, Kato K, Kawase D, Kinoshita N, et al. Tenascin-C concentration in synovial fluid correlates with radiographic progression of knee osteoarthritis. J Rheumatol 2004;31:2021–2026.
Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am 1971;53:523–537.
Pfander D, Swoboda B, Kirsch T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am J Pathol 2001;159:1777–1783.
Masuda K, Takegmi K, An H, Kumano F, Chiba K, Andersson GB, et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res 2003;21:922–930.
Jarvinen TA, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Hurme T, et al. Mechanical loading regulates tenascin-C expression in the osteotendinous junction. J Cell Sci 1999;112:3157–3166.
Chiquet-Ehrismann R, Tannheimer M, Koch M, Brunner A, Spring J, Martin D, et al. Tenascin-C expression by fibroblasts is elevated in stressed collagen gels. J Cell Biol 1994;127:2093–2101.
Cowan KN, Jones PL, Rabinovitch M. Regression of hypertrophied rat pulmonary arteries in organ culture is associated with suppression of proteolytic activity, inhibition of tenascin-C, and smooth muscle apoptosis. Circ Res 1999;84:1223–1233.
Savarese JJ, Erickson H, Scully SP. Articular chondrocyte tenascin-C production and assembly into de novo extracellular matrix. J Orthop Res 1996;14:273–281.
Pottenger LA, Lyon NB, Hecht JD, Neustadt PM, Robinson RA. Influence of cartilage particle size and proteoglycan aggregation on immobilization of proteoglycans. J Biol Chem 1982;257:11479–11485.
Day JM, Olin AI, Murdoch AD, Canfield A, Sasaki T, Timpl R, et al. Alternative splicing in the aggrecan G3 domain influences binding interactions with tenascin-C and other extracellular matrix proteins. J Biol Chem 2004;279:12 511–12 518.
Lee DA, Noguchi T, Knight MM, O’Donnell L, Bentley G, Bader DL. Response of chondrocyte subpopulations cultured within unloaded and loaded agarose. J Orthop Res 1998;16:726–733.
Wong M, Wuethrich P, Eggli P, Hunziker E. Zone-specific cell biosynthetic activity in mature bovine articular cartilage: a new method using confocal microscopic stereology and quantitative autoradiography. J Orthop Res 1996;14:424–432.
Murphy LI, Fischer D, Chiquet-Ehrismann R, Mackie EJ. Tenascin-C induced stimulation of chondrogenesis is dependent on the presence of the C-terminal fibrinogen-like globular domain. FEBS Lett 2000;480:189–192.
Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev 1992;6:1821–1831.
Forsberg E, Hirsch E, Frohlich L, Meyer M, Ekblom P, Aszodi A, et al. Skin wounds and severed nerves heal normally in mice lacking tenascin-C. Proc Natl Acad Sci USA 1996;93:6594–6599.
Hunziker EB, Driesang IM, Morris EA. Chondrogenesis in cartilage repair is induced by members of the transforming growth factor-beta superfamily. Clin Orthop Relat Res 2001;391:171–181.
Tucker RP, Hammarback JA, Jenrath DA, Mackie EJ, Xu Y. Tenascin expression in the mouse: in situ localization and induction in vitro by bFGF. J Cell Sci 1993;104:69–76.
Pearson CA, Pearson D, Shibahara S, Hofsteenge J, Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGFbeta. EMBO J 1988;7:2977–2982.
Sakai T, Kawakatsu H, Ohta J, Saito M. Tenascin induction in tenascin nonproducing carcinoma cell lines in vivo and by TGFbeta 1 in vitro. J Cell Physiol 1994;159:561–572.
Author information
Authors and Affiliations
About this article
Cite this article
Nakoshi, Y., Hasegawa, M., Akeda, K. et al. Distribution and role of tenascin-C in human osteoarthritic cartilage. J Orthop Sci 15, 666–673 (2010). https://doi.org/10.1007/s00776-010-1513-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-010-1513-x