Abstract
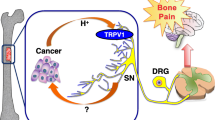
Bone pain is one of the most common and life-limiting complications of cancer metastasis to bone. Although the mechanism of bone pain still remains poorly understood, bone pain is evoked as a consequence of sensitization and excitation of sensory nerves (SNs) innervating bone by noxious stimuli produced in the microenvironment of bone metastases. We showed that bone is innervated by calcitonin gene-related protein (CGRP)+ SNs extending from dorsal root ganglia (DRG), the cell body of SNs, in mice. Mice intratibially injected with Lewis lung cancer (LLC) cells showed progressive bone pain evaluated by mechanical allodynia and flinching with increased CGRP+ SNs in bone and augmented SN excitation in DRG as indicated by elevated numbers of pERK- and pCREB-immunoreactive neurons. Immunohistochemical examination of LLC-injected bone revealed that the tumor microenvironment is acidic. Bafilomycin A1, a selective inhibitor of H+ secretion from vacuolar proton pump, significantly alleviated bone pain, indicating that the acidic microenvironment contributes to bone pain. We then determined whether the transient receptor potential vanilloid 1 (TRPV1), a major acid-sensing nociceptor predominantly expressed on SNs, plays a role in bone pain by intratibially injecting LLC cells in TRPV1-deficient mice. Bone pain and SN excitation in the DRG and spinal dorsal horn were significantly decreased in TRPV1−/− mice compared with wild-type mice. Our results suggest that TRPV1 activation on SNs innervating bone by the acidic cancer microenvironment in bone contributes to SN activation and bone pain. Targeting acid-activated TRPV1 is a potential therapeutic approach to cancer-induced bone pain.






Similar content being viewed by others
References
Weilbaecher KN, Guise TA, McCauley LK (2011) Cancer to bone: a fatal attraction. Nat Rev Cancer 11:411–425
Randall RL (2014) A promise to our patients with metastatic bone disease. Ann Surg Oncol 21:4049–4050
Coleman RE, Gregory W, Marshall H, Wilson C, Holen I (2013) The metastatic microenvironment of breast cancer: clinical implications. Breast 22:S50–S56
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12:6243s–6249s
Saad F, Ivanescu C, Phung D, Loriot Y, Abhyankar S, Beer TM, Tombal B, Holmstrom S (2017) Skeletal-related events significantly impact health-related quality of life in metastatic castration-resistant prostate cancer: data from PREVAIL and AFFIRM trials. Prostate Cancer Prostatic Dis 20:110–116
von Moos R, Costa L, Ripamonti CI, Niepel D, Santini D (2017) Improving quality of life in patients with advanced cancer: targeting metastatic bone pain. Eur J Cancer 71:80–94
Mercadante S (1997) Malignant bone pain: pathophysiology and treatment. Pain 69:1–18
Mantyh P (2013) Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 154:S54–S62
Falk S, Dickenson AH (2014) Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol 32:1647–1654
Dalal S, Bruera E (2013) Access to opioid analgesics and pain relief for patients with cancer. Nat Rev Clin Oncol 10:108–116
Yoneda T, Hiasa M, Nagata Y, Okui T, White FA (2015) Acidic microenvironment and bone pain in cancer-colonized bone. Bonekey Rep 4:690
Yoneda T, Hiasa M, Nagata Y, Okui T, White F (2015) Contribution of acidic extracellular microenvironment of cancer-colonized bone to bone pain. Biochim Biophys Acta 1848:2677–2684
Patrick DL, Cleeland CS, von Moos R, Fallowfield L, Wei R, Ohrling K, Qian Y (2015) Pain outcomes in patients with bone metastases from advanced cancer: assessment and management with bone-targeting agents. Support Care Cancer 23:1157–1168
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593
Yoneda T, Tanaka S, Hata K (2013) Role of RANKL/RANK in primary and secondary breast cancer. World J Orthop 4:178–185
Qin A, Cheng TS, Pavlos NJ, Lin Z, Dai KR, Zheng MH (2012) V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int J Biochem Cell Biol 44:1422–1435
Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ (2006) Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res 66:5216–5223
Cotter K, Capecci J, Sennoune S, Huss M, Maier M, Martinez-Zaguilan R, Forgac M (2015) Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells. J Biol Chem 290:3680–3692
Parks SK, Chiche J, Pouyssegur J (2013) Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer 13:611–623
Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y, Yasui N, Yoneda T (2011) The a3 isoform vacuolar type H(+)-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res 9:845–855
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284
Krames ES (2015) The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation 18:24–32 (discussion 32)
Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW (2013) Cancer-induced bone pain: mechanisms and models. Neurosci Lett 557 Pt A:52–59
Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29:355–384
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C, Dillin A (2014) TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 157:1023–1036
Isono M, Suzuki T, Hosono K, Hayashi I, Sakagami H, Uematsu S, Akira S, DeClerck YA, Okamoto H, Majima M (2011) Microsomal prostaglandin E synthase-1 enhances bone cancer growth and bone cancer-related pain behaviors in mice. Life Sci 88:693–700
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110
Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O’Reilly T, Wotherspoon G, Winter J, Green J, Urban L (2002) A rat model of bone cancer pain. Pain 96:129–140
Slosky LM, Largent-Milnes TM, Vanderah TW (2015) Use of animal models in understanding cancer-induced bone pain. Cancer Growth Metastasis 8:47–62
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Shimoyama M, Tanaka K, Hasue F, Shimoyama N (2002) A mouse model of neuropathic cancer pain. Pain 99:167–174
Ivanusic JJ (2009) Size, neurochemistry, and segmental distribution of sensory neurons innervating the rat tibia. J Comp Neurol 517:276–283
Nakanishi M, Hata K, Nagayama T, Sakurai T, Nishisho T, Wakabayashi H, Hiraga T, Ebisu S, Yoneda T (2010) Acid activation of Trpv1 leads to an up-regulation of calcitonin gene-related peptide expression in dorsal root ganglion neurons via the CaMK-CREB cascade: a potential mechanism of inflammatory pain. Mol Biol Cell 21:2568–2577
Hiraga T, Myoui A, Hashimoto N, Sasaki A, Hata K, Morita Y, Yoshikawa H, Rosen CJ, Mundy GR, Yoneda T (2012) Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer Res 72:4238–4249
Verlander JW, Madsen KM, Larsson L, Cannon JK, Tisher CC (1989) Immunocytochemical localization of intracellular acidic compartments: rat proximal nephron. Am J Physiol 257:F454–F462
Serre CM, Farlay D, Delmas PD, Chenu C (1999) Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25:623–629
Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW (2002) Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113:155–166
Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, Mantyh PW (2010) A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone 46:306–313
Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S et al (2013) Sema3A regulates bone-mass accrual through sensory innervations. Nature 497:490–493
Benemei S, Nicoletti P, Capone JG, Geppetti P (2009) CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol 9:9–14
Mantyh PW (2014) The neurobiology of skeletal pain. Eur J Neurosci 39:508–519
Hiasa M, Okui T, Allette YM, Ripsch MS, Sun-Wada GH, Wakabayashi H, Roodman GD, White FA, Yoneda T (2017) Bone pain induced by multiple myeloma is reduced by targeting V-ATPase and ASIC3. Cancer Res 77:1283–1295
Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T (2006) Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone 39:1107–1115
Doya H, Ohtori S, Takahashi K, Aoki Y, Ino H, Takahashi Y, Moriya H, Yamashita T (2005) Extracellular signal-regulated kinase mitogen-activated protein kinase activation in the dorsal root ganglion (DRG) and spinal cord after DRG injury in rats. Spine (Phila Pa 1976) 30:2252–2256
Coggeshall RE (2005) Fos, nociception and the dorsal horn. Prog Neurobiol 77:299–352
Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW (2005) Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci 25:3126–3131
Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A (2007) Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience 148:560–572
Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A (2009) SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth 102:251–258
Fuseya S, Yamamoto K, Minemura H, Yamaori S, Kawamata T, Kawamata M (2016) Systemic QX-314 reduces bone cancer pain through selective inhibition of transient receptor potential vanilloid subfamily 1-expressing primary afferents in mice. Anesthesiology 125:204–218
Acknowledgements
This study was supported by the Princess Takamatsu Cancer Research Foundation (08-24020) and Naito Memorial Foundation of Science Promotion (200-07) to TY and the Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (17014058, 23390422, 23659870, 26293394, 26670806) to TY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Wakabayashi, H., Wakisaka, S., Hiraga, T. et al. Decreased sensory nerve excitation and bone pain associated with mouse Lewis lung cancer in TRPV1-deficient mice. J Bone Miner Metab 36, 274–285 (2018). https://doi.org/10.1007/s00774-017-0842-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0842-7