Abstract
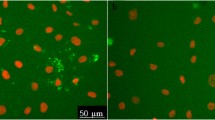
Melittin, from the honeybee venom, is a membrane active protein, whose cytotoxicity to human endothelial cells has not been described yet. In this work, we studied its time-dependent cytotoxicity on human umbilical vein endothelial cells (HUVECs). Since HUVECs grow in culture as adherent cells, suspension of cells is required before measuring cytotoxicity with a haemocytometer or flow cytometry. Therefore, we also tried to discover whether the result of cytotoxicity tests of melittin is influenced by the preparation of the cell suspension. For this purpose, we compared the results of haemocytometer-based trypan blue assay and flow cytometry using 7-aminoactinomycin D (7-AAD) with results of fluorescence microscopy using 7-AAD and 4', 6-diamidino-2-phenylindole (DAPI). Melittin over 60 min exposure evoked a rapid decline in the survival of HUVEC. After 60 min exposure to melittin, the phase contrast microscopy demonstrated massive necrosis in the remaining attached cells. Fluorescence microscopy detected both viable and non-viable cells in adequate proportions at all exposure times, whereas haemocytometer-based assay and flow cytometry highly underestimated the percentage of non-viable cells or even failed to detect any dead cells. Our data clearly indicate that the induction of large-scale damage to adherent endothelial cells by melittin results in a loss of the majority of necrotic cells during sample preparation for flow cytometry or a haemocytometer-based assay. In the case of adherent cell culture, therefore, fluorescence microscopy was shown to be a more appropriate method for quantitative analysis of cell death caused by a fast-acting cytolytic toxin such as melittin.




Similar content being viewed by others
References
Alqutub AN, Masoodi I, Alsayari K, Alomair A (2011) A bee sting therapy-induced hepatotoxicity: a case report. World J Hepatol 3:268–270
Batista U, Garvas M, Nemec M, Schara M, Veranic P, Koklic T (2010) Effects of different detachment procedures on viability, nitroxide reduction kinetics and plasma membrane heterogeneity of V-79 cells. Cell Biol Int 34:663–668
Brown TC, Tankersley MS (2011) The sting of the honeybee: an allergic perspective. Ann Allergy Asthma Immunol 107:463–470
Cerne K, Kristan KC, Budihna MV, Stanovnik L (2010) Mechanisms of changes in coronary arterial tone induced by bee venom toxins. Toxicon 56:305–312
Coder DM (2001) Assessment of cell viability. In: Robinson JP (ed) Current protocols in cytometry. Wiley, New York, pp 9.2.1–9.2.14
Erman A, Veranic P (2010) Time- and temperature-dependent autolysis of urinary bladder epithelium during ex vivo preservation. Protoplasma 246:81–87
Franca FO, Benvenuti LA, Fan HW, Dos Santos DR, Hain SH, Picchi-Martins FR, Cardoso JL, Kamiguti AS, Theakston RD, Warrell DA (1994) Severe and fatal mass attacks by “killer” bees (Africanized honey bees—Apis mellifera scutellata) in Brazil: clinicopathological studies with measurement of serum venom concentrations. The Q J Med 87:269–282
Grisotto LS, Mendes GE, Castro I, Baptista MA, Alves VA, Yu L, Burdmann EA (2006) Mechanisms of bee venom-induced acute renal failure. Toxicon 48:44–54
Hacker S, Messer WS, Bachman KA (2009) Pharmacology: principles and Practice. Academic, London
Killion JJ, Dunn JD (1986) Differential cytolysis of murine spleen, bone-marrow and leukemia cells by melittin reveals differences in membrane topography. Biochem Biophys Res Commun 139(1):222–227
Lo WC, Henk WG, Enright FM (1997) Light-microscopic studies of 3T3 cell plasma membrane alternation mediated by melittin. Toxicon 35:12–26
Maher S, McClean S (2008) Melittin exhibits necrotic cytotoxicity in gastrointestinal cells which is attenuated by cholesterol. Biochem Pharmacol 75(5):1104–1114
Maher S, McClean S (2006) Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem Pharmacol 71:1289–1298
Okamoto T, Isoda H, Kubota N, Takahata K, Takahashi T, Kishi T, Nakamura TY, Muromachi Y, Matsui Y, Goshima K (1995) Melittin cardiotoxicity in cultured mouse cardiac myocytes and its correlation with calcium overload. Toxicol Appl Pharmacol 133:150–163
Ownby CL, Powell JR, Jiang MS, Fletcher JE (1997) Melittin and phospholipase A2 from bee (Apis mellifera) venom cause necrosis of murine skeletal muscle in vivo. Toxicon 35:67–80
Pan H, Soman NR, Schlesinger PH, Lanza GM, Wickline SA (2011) Cytolytic peptide nanoparticles (‘NanoBees’) for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 3:318–327
Pratt JP, Ravnic DJ, Huss HT, Jiang X, Orozco BS, Mentzer SJ (2005) Melittin-induced membrane permeability: a nonosmotic mechanism of cell death. In Vitro Cell Dev Biol Anim 41:349–355
Raghuraman H, Chattopadhyay A (2007) Melittin: a membrane-active peptide with diverse functions. Biosci Rep 27:189–223
Rapoport RM, Ashraf M, Murad F (1989) Effects of melittin on endothelium-dependent relaxation and cyclic GMP levels in rat aorta. Circ Res 64:463–473
Schumacher MJ, Egen NB (1995) Significance of Africanized bees for public health. A Review Arch Intern Med 155:2038–2043
Shaposhnikova VV, Egorova MV, Kudryavtsev AA, Levitman M, Korystov YN (1997) The effect of melittin on proliferation and death of thymocytes. FEBS let 410:285–288
Strober W (2001) Trypan blue exclusion test of cell viability. In: Coligan JE (ed) Current protocols in immunology. Wiley, New York, 21:A. 3B.1. - A. 3B.2
Su M, He C, West CA, Mentzer SJ (2001) Cytolytic peptides induce biphasic permeability changes in mammalian cell membranes. J Immunol Methods 252:63–71
Tarnowski BI, Spinale FG, Nicholson JH (1991) DAPI as a useful stain for nuclear quantitation. Biotech Histochem 66:297–302
Tejuca M, Anderluh G, Dalla Serra M (2009) Sea anemone cytolysins as toxic components of immunotoxins. Toxicon 54:1206–1214
Tosteson M, Holmes SJ, Razin M, Tosteson DC (1985) Melittin lysis of red cells. J Membr Biol 87:35–44
Weston KM, Raison RL (1998) Interaction of melittin with a human lymphoblastoid cell line, HMy2. J Cell Biochem 68:164–173
Weston KM, Alsalami M, Raison RL (1994) Cell membrane changes induced by the cytolytic peptide, melittin, are detectable by 90 degrees laser scatter. Cytometry 15:141–147
Acknowledgments
This work was supported by research grants from the Ministry of Higher Education, Science and Technology (P3-067, P3-0108). We thank Mr. Martin Cregeen for checking the English of the text.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: DAMJANA Drobne
Rights and permissions
About this article
Cite this article
Černe, K., Erman, A. & Veranič, P. Analysis of cytotoxicity of melittin on adherent culture of human endothelial cells reveals advantage of fluorescence microscopy over flow cytometry and haemocytometer assay. Protoplasma 250, 1131–1137 (2013). https://doi.org/10.1007/s00709-013-0489-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0489-8