Abstract
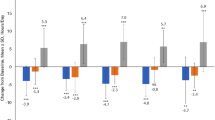
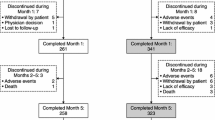
The study objective was to assess the efficacy, safety and feasibility of switching from levodopa/benserazide (LB) or levodopa/carbidopa (LC) to levodopa/carbidopa/entacapone (LCE) in Parkinson’s disease (PD) patients with wearing-off. This was a multicenter, open-label, 6-week study; the primary outcome was success rate based on the patient-assessed Clinical Global Impression of Change (P-CGI-C). Secondary outcomes included investigator-assessed CGI-C (I-CGI-C), change from baseline in Unified Parkinson’s Disease Rating Scale (UPDRS), motor/non-motor wearing-off symptoms and quality of life–visual analog scale (QoL-VAS). After switching to LCE, 77% of patients reported an ‘improvement’ (p < 0.0001 vs. patients reporting ‘no change or worsening’). Significant improvements were seen in I-CGI-C, UPDRS and QoL-VAS, regardless of prior therapy. Oral levodopa dosing was increased in 28% of patients; the primary outcome remained significant when these patients were excluded. The data suggest that switching from LB/LC to LCE provided a significant benefit in PD patients with wearing-off.



Similar content being viewed by others
References
Brooks DJ, Sagar H (2003) Entacapone is beneficial in both fluctuating and non-fluctuating patients with Parkinson’s disease: a randomised, placebo controlled, double blind, six month study. J Neurol Neurosurg Psychiatry 74:1071–1079
Brooks DJ, Agid Y, Eggert K, Widner H, Ostergaard K, Holopainen A, The TC-INIT Study Group (2005) Treatment of end-of-dose wearing-off in Parkinson’s disease: Stalevo® (Levodopa/Carbidopa/Entacapone) and levodopa/DDCI given in combination with Comtess®/Comtan® (Entacapone) provide equivalent improvements in symptom control superior to that of traditional levodopa/DDCI treatment. Eur Neurol 53:197–202
de la Fuente-Fernandez R, Stoessl AJ (2004) The biochemical bases of the placebo effect. Sci Eng Ethics 10:143–150
de la Fuente-Fernandez R, Schulzer M, Stoessl AJ (2004) Placebo mechanisms and reward circuitry: clues from Parkinson’s disease. Biol Psychiatry 56:67–71
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170
Gordin A, Kaakkola S, Teravainen H (2004) Clinical advantages of COMT inhibition with entacapone––a review. J Neural Transm 111:1343–1363
Koller W, Guarnieri M, Hubble J, Rabinowicz AL, Silver D (2005) An open-label evaluation of the tolerability and safety of Stalevo® (carbidopa, levodopa and entacapone) in Parkinson’s disease patients experiencing wearing-off. J Neural Transm 112:221–230
Kuoppamäki M, Korpela K, Marttila R, Kaasinen V, Hartikainen P, Lyytinen J, Kaakkola S, Hänninen J, Löyttyniemi E, Kailajärvi M, Ruokoniemi P, Ellmén J (2009) Comparison of pharmacokinetic profile of levodopa throughout the day between levodopa/carbidopa/entacapone and levodopa/carbidopa when administered four- or five-times daily. Eur J Clin Pharmacol 65:443–455
Linazasoro G, Kulisevsky J, Hernandez B (2008) Should levodopa dose be reduced when switched to stalevo? Eur J Neurol 15:257–261
Muller T, Erdmann C, Muhlack S, Bremen D, Przuntek H, Goetze O, Woitalla D (2006a) Pharmacokinetic behaviour of levodopa and 3-O-methyldopa after repeat administration of levodopa/carbidopa with and without entacapone in patients with Parkinson’s disease. J Neural Transm 113:1441–1448
Muller T, Erdmann C, Muhlack S, Bremen D, Przuntek H, Woitalla D (2006b) Inhibition of catechol-O-methyltransferase contributes to more stable levodopa plasma levels. Mov Disord 21:332–336
Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonuccelli U, Damier P, De Yebenes J, Gershanik O, Guttman M, Grandas F, Hallett M, Hornykiewicz O, Jenner P, Katzenschlager R, Langston WJ, LeWitt P, Melamed E, Mena MA, Michel PP, Mytilineou C, Obeso JA, Poewe W, Quinn N, Raisman-Vozari R, Rajput AH, Rascol O, Sampaio C, Stocchi F (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19:997–1005
Parkinson Study Group (1997) Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Ann Neurol 42:747–755
Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M (2002) Efficacy and safety of entacapone in Parkinson’s disease patients with suboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurologica Scand 105:245–255
Rinne UK, Larsen JP, Siden A, Worm-Petersen J, The Nomecomt Study Group (1998) Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Neurology 51:1309–1314
Stacy M, Hauser R, Oertel W, Schapira A, Sethi K, Stocchi F, Tolosa E (2006) End-of-dose wearing off in Parkinson disease: a 9-question survey assessment. Clin Neuropharmacol 29:312–321
Stocchi F (2006) The levodopa wearing-off phenomenon in Parkinson’s disease: pharmacokinetic considerations. Expert Opin Pharmacother 7:1399–1407
Acknowledgments
This study was funded by Orion Corporation Orion Pharma. The authors would like to thank Diya Lahiri, Ph.D. for her editorial assistance.
Conflict of interest statement
Karla Eggert has worked as a consultant for Orion Pharma, Schwarz Pharma Neuroscience (UCB), Solvay Pharmaceuticals, Valeant Pharmaceuticals International, Desitin, Orphane Europe, Boehringer Ingelheim, GlaxoSmithKline. She receives scientific grants from the German Ministry of Education and Health, from the Pitzer Foundation and from the Rhön Foundation; Helena Nissinen, Mikko Kuoppamäki and Liisa Luotonen are employees of Orion Pharma; Örjan Skogar and Khaled Amar have acted as consultants for Orion Pharma; statistical analysis was carried out my Mika Leinonen who has served as a consultant for Orion Pharma; W. H. Oertel has worked as a consultant for Boehringer Ingelheim, EISAI Medical Research, GE Health, GlaxoSmithKline, Novartis, Orion Pharma, Schwarz Pharma Neuroscience (UCB), Solvay Pharmaceuticals, Teva Neuroscience, Valeant Pharmaceuticals International. Prof. Dr. W. H. Oertel and his collaborators have received grants from Boehringer Ingelheim, Hoffmann LaRoche, Schwarz Pharma Neuroscience for basic science research and educational support. He receives scientific grants from the German Ministry of Education and Health, from the German Research Foundation, from the Pitzer Foundation and from the Rhön Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the SENSE study group.
Appendix
Appendix
The members of the SENSE Study Group are: Khaled Amar, The Royal Bournemouth Hospital, Bournemouth, UK; Ralf Bodenschatz, Pharmakologisches Studienzentrum Chemnitz, Chemnitz, Germany; Carsten Buhmann, Universitätskrankenhaus Eppendorf, Hamburg, Germany; Sven-Erik Bysell, Visby lasarett, Visby, Sweden; Ilona Csoti, Parkinson-Zentrum Gertrudis-Klinik, Leun-Biskirchen, Germany; Paul Davies, Northampton General Hospital, Northampton, UK; Karla Eggert, Klinik für Neurologie, Philipps-Universität Marburg, Germany; Christer Ewaldsson, Neurologmottagningen, Västra Frölunda, Sweden; Duncan Forsyth, Addenbrookes Hospital, Cambridge, UK; Thomas Gasser, Eberhard-Karls-Universität, Tübingen, Germany; Jan Kassubek, Neurologische Klinik der Universität Ulm, Ulm, Germany; Andreas Kupsch, Humboldt Universität Charité Neurologische Klinik, Berlin, Germany; Nelson Lo, Leicester General Hospital, Leicester, UK; Wolfhard Lünser, Facharzt für Neurologie und Neurochirurgie, Hamm, Germany; Thomas Müller, St. Josef-Hospital Klinikum der Ruhr-Universität-Bochum, Bochum, Germany; Alexander Nass, Nervenarztpraxis, Köln, Germany; Udo Polzer, Asklepios Fachklinikum Stadtroda, Stadtroda, Germany; Gerd Reifschneider, Gemeinschaftspraxis für Neurologie & Psychiatrie, Erbach, Germany; Stuart Rochow, Woodend Hospital, Aberdeen, UK; Anirban Saha, Royal Sussex County Hospital, Brighton, UK; Christine Schuster, Neurologische Praxis, Giessen, Germany; Örjan Skogar, Länssjukhuset Ryhov, Jönköping, Sweden; Alexander Storch, Technische Universität Dresden, Dresden, Germany; Claudia Trenkwalder, Paracelsus-Elena Klinik, Kassel, Germany; Wolfgang Oertel, Klinik für Neurologie, Philipps-Universität Marburg, Germany; Hans-Jürgen von Giesen, Alexianer-Krankenhaus, Krefeld, Germany; Görel Wachtmeister, Nyköpings Lasarett, Nyköping, Sweden; Richard Walker, North Tyneside General Hospital, North Shields, UK; Ulf Wigow, Höglandssjukhuset i Nässjö, Nässjö, Sweden.
Rights and permissions
About this article
Cite this article
Eggert, K., Skogar, Ö., Amar, K. et al. Direct switch from levodopa/benserazide or levodopa/carbidopa to levodopa/carbidopa/entacapone in Parkinson’s disease patients with wearing-off: efficacy, safety and feasibility—an open-label, 6-week study. J Neural Transm 117, 333–342 (2010). https://doi.org/10.1007/s00702-009-0344-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0344-4