Abstract
Background
The purpose of the present study was to clarify the characteristics of endothelial cell (EC) proliferation in intraplaque microvessels in vulnerable plaques and impact on clinical results.
Methods
The present study included 76 patients who underwent carotid endarterectomy. Patients were classified into three groups based on their symptoms: asymptomatic, symptomatic without recurrent ischemic event, and symptomatic with recurrent ischemic event. MR plaque imaging was performed and surgical specimens underwent immunohistochemical analysis. The number of CD31+ microvessels, and Ki67+ and CD105+ ECs in the carotid plaques was quantified, as measurements of maximum CD31+ microvessel diameter.
Results
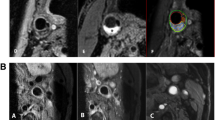
MR plaque imaging yielded 41 subjects (54.0%) diagnosed with plaque with intraplaque hemorrhage (IPH), 14 subjects (18.4%) diagnosed with fibrous plaques, and 21 (27.6%) subjects diagnosed with lipid-rich plaques. The average largest diameter of microvessel in fibrous plaques, lipid-rich plaques, and plaque with IPH was 12.7 ± 4.1 μm, 31.3 ± 9.3 μm, and 56.4 ± 10.0 μm, respectively (p < 0.01). Dilated microvessels (>40 μm) were observed in 9.6% of plaques with IPH but only in 2.8% of lipid-rich plaques and 0% of fibrous plaques (p < 0.01). Ki67+/CD31+ ECs were identified in 2.8 ± 1.2% of fibrous plaques, 9.6 ± 6.9% of lipid-rich plaques, and in 19.5 ± 5.9% of plaques with IPH (p < 0.01). The average largest diameter of microvessels in the asymptomatic group was 17.1 ± 8.7 μm, 32.3 ± 10.8 μm in the symptomatic without recurrence group, and 55.2 ± 13.2 μm in the symptomatic with recurrence group (p < 0.01).
Conclusion
Dilated microvessels with proliferative ECs may play a key role in IPH pathogenesis. Furthermore, dilated microvessels are likely related to clinical onset and the recurrence of ischemic events. The purpose of the present study was to clarify the characteristics of EC proliferation in intraplaque microvessels in vulnerable plaques and their impact on clinical results, focusing on dilated intraplaque microvessels.






Similar content being viewed by others
References
Altaf N, MacSweeney ST, Gladman J, Auer DP (2007) Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 38:1633–1635. https://doi.org/10.1161/STROKEAHA.106.473066
Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, Burn J, Diamond AG (2000) Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol 217:42–53. https://doi.org/10.1006/dbio.1999.9534
Barbara NP, Wrana JL, Letarte M (1999) Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem 274:584–594. https://doi.org/10.1074/jbc.274.2.584
Barnett JM, Suarez S, McCollum GW, Penn JS (2014) Endoglin promotes angiogenesis in cell- and animal-based models of retinal neovascularization. Invest Ophthalmol Vis Sci 55:6490–6498. https://doi.org/10.1167/iovs.14-14945
Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M (1992) Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem 267:19027–19030
de Vries MR, Quax PH (2016) Plaque angiogenesis and its relation to inflammation and atherosclerotic plaque destabilization. Curr Opin Lipidol 27:499–506. https://doi.org/10.1097/MOL.0000000000000339
Guo M, Cai Y, Yao X, Li Z (2018) Mathematical modeling of atherosclerotic plaque destabilization: role of neovascularization and intraplaque hemorrhage. J Theor Biol 450:53–65. https://doi.org/10.1016/j.jtbi.2018.04.031
Jaipersad AS, Lip GY, Silverman S, Shantsila E (2014) The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol 63:1–11. https://doi.org/10.1016/j.jacc.2013.09.019
Kashiwazaki D, Akioka N, Kuwayama N, Noguchi K, Tanaka K, Kuroda S (2015) Pathophysiology of acute cerebrovascular syndrome in patients with carotid artery stenosis: a magnetic resonance imaging/single-photon emission computed tomography study. Neurosurgery 76:427–433; discussion 433-424. https://doi.org/10.1227/NEU.0000000000000655
Kashiwazaki D, Akioka N, Kuwayama N, Hayashi T, Noguchi K, Tanaka K, Kuroda S (2016) Involvement of circulating endothelial progenitor cells in carotid plaque growth and vulnerability. J Neurosurg 125:1549–1556. https://doi.org/10.3171/2015.10.JNS151500
Kashiwazaki D, Koh M, Uchino H, Akioka N, Kuwayama N, Noguchi K, Kuroda S (2018) Hypoxia accelerates intraplaque neovascularization derived from endothelial progenitor cells in carotid stenosis. J Neurosurg:1–8. https://doi.org/10.3171/2018.4.JNS172876
Kelly PJ, Camps-Renom P, Giannotti N, Marti-Fabregas J, Murphy S, McNulty J, Barry M, Barry P, Calvet D, Coutts SB, Cronin S, Delgado-Mederos R, Dolan E, Fernandez-Leon A, Foley S, Harbison J, Horgan G, Kavanagh E, Marnane M, McDonnell C, O'Donohoe M, Sharma V, Walsh C, Williams D, O'Connell M (2019) Carotid plaque inflammation imaged by (18)F-fluorodeoxyglucose positron emission tomography and risk of early recurrent stroke. Stroke 50:1766–1773. https://doi.org/10.1161/STROKEAHA.119.025422
Leroyer AS, Rautou PE, Silvestre JS, Castier Y, Leseche G, Devue C, Duriez M, Brandes RP, Lutgens E, Tedgui A, Boulanger CM (2008) CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J Am Coll Cardiol 52:1302–1311. https://doi.org/10.1016/j.jacc.2008.07.032
Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP (1999) Defective angiogenesis in mice lacking endoglin. Science 284:1534–1537. https://doi.org/10.1126/science.284.5419.1534
Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, Kumar S (2003) CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci 116:2677–2685. https://doi.org/10.1242/jcs.00470
Liu XS, Zhao HL, Cao Y, Lu Q, Xu JR (2012) Comparison of carotid atherosclerotic plaque characteristics by high-resolution black-blood MR imaging between patients with first-time and recurrent acute ischemic stroke. AJNR Am J Neuroradiol 33:1257–1261. https://doi.org/10.3174/ajnr.A2965
Lu M, Peng P, Cui Y, Qiao H, Li D, Cai J, Zhao X (2018) Association of progression of carotid artery wall volume and recurrent transient ischemic attack or stroke: a magnetic resonance imaging study. Stroke 49:614–620. https://doi.org/10.1161/STROKEAHA.117.019422
Marioni G, Marino F, Giacomelli L, Staffieri C, Mariuzzi ML, Violino E, De Filippis C (2006) Endoglin expression is associated with poor oncologic outcome in oral and oropharyngeal carcinoma. Acta Otolaryngol 126:633–639. https://doi.org/10.1080/00016480500452558
Marioni G, D'Alessandro E, Giacomelli L, Staffieri A (2010) CD105 is a marker of tumour vasculature and a potential target for the treatment of head and neck squamous cell carcinoma. J Oral Pathol Med 39:361–367. https://doi.org/10.1111/j.1600-0714.2010.00888.x
Martin-Padura I, De Castellarnau C, Uccini S, Pilozzi E, Natali PG, Nicotra MR, Ughi F, Azzolini C, Dejana E, Ruco L (1995) Expression of VE (vascular endothelial)-cadherin and other endothelial-specific markers in haemangiomas. J Pathol 175:51–57. https://doi.org/10.1002/path.1711750109
Michel JB, Martin-Ventura JL, Nicoletti A, Ho-Tin-Noe B (2014) Pathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis 234:311–319. https://doi.org/10.1016/j.atherosclerosis.2014.03.020
Munoz-Chapuli R, Quesada AR, Angel Medina M (2004) Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci 61:2224–2243. https://doi.org/10.1007/s00018-004-4070-7
Pages G, Pouyssegur J (2005) Transcriptional regulation of the vascular endothelial growth factor gene—a concert of activating factors. Cardiovasc Res 65:564–573. https://doi.org/10.1016/j.cardiores.2004.09.032
Parma L, Baganha F, Quax PHA, de Vries MR (2017) Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur J Pharmacol 816:107–115. https://doi.org/10.1016/j.ejphar.2017.04.028
Saito A, Sasaki M, Ogasawara K, Kobayashi M, Hitomi J, Narumi S, Ohba H, Yamaguchi M, Kudo K, Terayama Y (2012) Carotid plaque signal differences among four kinds of T1-weighted magnetic resonance imaging techniques: a histopathological correlation study. Neuroradiology 54:1187–1194. https://doi.org/10.1007/s00234-012-1025-9
Schmidt D, von Hochstetter AR (1995) The use of CD31 and collagen IV as vascular markers. A study of 56 vascular lesions. Pathol Res Pract 191:410–414. https://doi.org/10.1016/S0344-0338(11)80727-2
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182:311–322. https://doi.org/10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9
Sedding DG, Boyle EC, Demandt JAF, Sluimer JC, Dutzmann J, Haverich A, Bauersachs J (2018) Vasa vasorum angiogenesis: key player in the initiation and progression of atherosclerosis and potential target for the treatment of cardiovascular disease. Front Immunol 9:706. https://doi.org/10.3389/fimmu.2018.00706
Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92:1355–1374. https://doi.org/10.1161/01.cir.92.5.1355
Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS (2005) Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 111:2768–2775. https://doi.org/10.1161/CIRCULATIONAHA.104.504167
Wang C, Wang Q, Gao W, Zhang Z, Lou Y, Jin H, Chen X, Lei B, Xu H, Mao C (2018) Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing. Acta Biomater 69:156–169. https://doi.org/10.1016/j.actbio.2018.01.019
Xu Y, Yuan C, Zhou Z, He L, Mi D, Li R, Cui Y, Wang Y, Wang Y, Liu G, Zheng Z, Zhao X (2016) Co-existing intracranial and extracranial carotid artery atherosclerotic plaques and recurrent stroke risk: a three-dimensional multicontrast cardiovascular magnetic resonance study. J Cardiovasc Magnetic Resonance 18:90. https://doi.org/10.1186/s12968-016-0309-3
Yoshida K, Narumi O, Chin M, Inoue K, Tabuchi T, Oda K, Nagayama M, Egawa N, Hojo M, Goto Y, Watanabe Y, Yamagata S (2008) Characterization of carotid atherosclerosis and detection of soft plaque with use of black-blood MR imaging. AJNR Am J Neuroradiol 29:868–874. https://doi.org/10.3174/ajnr.A1015
Funding
D.K. provided financial support in the form of JSPS KAKENHI (grant number JP20K09342).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The sponsor had no role in the design or conduct of this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution of Toyama University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Comments
This is a fascinating study from the expert carotid group in Toyama. These authors have previously reported that microvessel formation and vessel dilatation were strongly associated with unstable plaques in human carotid stenosis. In the present submission, the investigators performed an immunohistochemical analysis of microvessels, endothelial cell (EC) proliferation in microvessels, endoglin, and hemoglobin in 76 surgically removed carotid plaques, specifically seeking to correlate intraplaque vessel morphology with MR imaging and clinical correlates. The results are fascinating and instructive. Dilated plaque microvessels were significantly correlated with clinical symptomatology and with intraplaque hemorrhage (IPH). Specifically, the results of the present study suggest that dilated microvessels (>40 μm) are accompanied by active proliferation of ECs in unstable plaques. Furthermore, the results also suggest that IPH is present in proximity to intraplaque dilated neovessels with EC proliferation in unstable carotid plaques. Other findings of note were that ECs in dilated microvessels were positive for CD105, a marker of cell proliferation often found in cells in pathological conditions, and that dilated intraplaque microvessels were correlated with symptom onset and recurrence. The authors demonstrate with authority that dilated microvessels are often accompanied by proliferative ECs and suggest that this could play a key role in developing IPH. High ratios of Ki67+/CD105+ ECs in dilated microvessels suggest that these dilated microvessels formed under pathological conditions. In addition, dilated microvessels are related to the clinical onset and recurrence of ischemic events in carotid stenosis.
This is a significant report that supports the rationale that plaque neovascularization and intraplaque hemorrhage (IPH) are important factors in plaque instability and clinically symptomatic carotid disease.
Christopher Miranda Loftus,
PA, USA
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery - Ischemia
Rights and permissions
About this article
Cite this article
Kashiwazaki, D., Yamamoto, S., Akioka, N. et al. Dilated microvessel with endothelial cell proliferation involves intraplaque hemorrhage in unstable carotid plaque. Acta Neurochir 163, 1777–1785 (2021). https://doi.org/10.1007/s00701-020-04595-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04595-0