Abstract
Objective
The present study was undertaken to reveal the influence of intracerebroventricular (ICV) benzamil on the dynamics of brain water accumulation in hyponatremic rats. Parameters of brain water homeostasis were continuously monitored, using in vivo magnetic resonance imaging (MRI) methods. The results were compared with those obtained in a previous study by tissue desiccation.
Methods
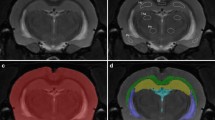
A 3-T MRI instrument was applied to perform serial diffusion-weighted imaging to measure the apparent diffusion coefficient (ADC) and MR spectroscopy to determine water signal. A decrease of ADC is thought to represent an increase of intracellular water, whereas water signal is used to quantify brain water content. Five groups of male Wistar rats were studied as follows: normonatremic, native animals (group NN, n = 7), hyponatremic animals (group HN, n = 8), hyponatremic animals treated with ICV benzamil (group HNB, n = 8), hyponatremic animals treated with ICV saline (group HNS, n = 5) and normonatremic animals treated with ICV benzamil (group NNB, n = 5). Hyponatremia was induced by intraperitoneal administration of 140 mmol/l dextrose solution in a dose of 20% of body weight. Benzamil hydrochloride (4 μg) was injected ICV to the treated animals.
Results
During the course of hyponatemia, ADC declined steadily from the baseline (100%) to reach a minimum of 92.32 ± 3.20% at 90 min (p < 0.0005). This process was associated with an increase in water signal to a maximum of 5.95 ± 2.62% at 100 min (p < 0.0005). After pretreatment with benzamil, no consistent changes occurred either in ADC or in water signal.
Conclusions
These findings suggest that sodium channel blockade with ICV benzamil has an immediate protective effect against the development of hyponatremic brain edema. Sodium channels, therefore, appear to be intimately involved in the initiation and progression of brain water accumulation in severe hyponatremia.


Similar content being viewed by others
References
Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A (2003) An α-syntrophindependent pool of Aqp-4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci USA 100:2106–2111
Amiry-Moghaddam M, Ottersen OP (2003) The molecular basis of water transport in the brain. Nat Rev Neurosci 4:991–1001
Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre P, Ottersen OP (2003) Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of α-syntrophin-null mice. Proc Natl Acad Sci USA 100:13615–13620
Aradi M, Steier R, Bukovics P, Szalay C, Perlaki G, Orsi G, Pál J, Janszky J, Dóczi T, Schwarcz A (2010) Quantitative proton MRI and MRS of the rat brain with a 3 T clinical scanner. [published online ahead of print Mar 22 2010]. J Neuroradiol http://www.ncbi.nlm.nih.gov/pubmed. PMID: 20334917
Bize V, Horisberger JD (2007) Sodium self-inhibition of human epithelial sodium channel: selectivity and affinity of the extracellular sodium sensing site. Am J Physiol Renal Physiol 293(4):F1137–F1146
Bugaj V, Pochynyuk O, Stockand JD (2009) Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol 297:F1411–F1418
Butterworth NB, Edinger RS, Frizzell RA, Johnson JP (2009) Regulation of epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol 296:F10–F24
Crowe WE, Ehrenfeld J, Brochiero E, Wills NK (1995) Apical membrane sodium and chloride entry during osmotic swelling of renal (A6) epithelial cells. J Membr Biol 144:81–91
Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS (1994) Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem 269:5497–5500
Hernando F, Choots O, Lolait SJ, Burbach JPH (2001) Immunohistochemical localization of the vasopressin V1B receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effect of vasopressin. Endocrinology 142:1659–1668
Junankar PR, Kirk K (2000) Organic osmolyte channels: a comparative view. Cell Physiol Biochem 10:355–360
Klatzo I (1994) Evolution of brain edema concepts. Acta Neurochir Suppl (Wien) 60:3–6
Liu X, Nakayama S, Amiry-Moghaddam M, Ottersen OP, Bhardwaj A (2010) Arginine-vasopressin V1 but not V2 receptor antagonism modulates infarct volume, brain water content and aquaporin-4 expression following experimental stroke. Neurocrit Care 12:124–131
Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163
Miyazaki H, Shiozaki A, Niisato N, Marunaka Y (2007) Physiological significance of hypotonicity–induced regulatory volume decrease: reduction in intracellular Cl-concentration acting as an intracellular signaling. Am J Physiol Renal Physiol 292:F1411–F1417
Niermann H, Amiry-Moghaddam M, Holthoff K, Witte OW, Ottersen OP (2001) A novel role of vasopressin in the brain: modulation of activity-dependent water flux in the neocortex. J Neurosci 21:3045–3051
Niisato N, Eaton BC, Marunaka Y (2004) Involvement of cytosolic Cl- in osmoregulation of α-ENaC gene expression. Am J Physiol Renal Physiol 287:F932–F939
Niisato N, Van Driessche W, Liu M, Marunaka Y (2000) Involvement of protein tyrosine kinase in osmoregulation of Na+ transport and membrane capacitance in renal A6 cells. J Membr Biol 175:63–77
Nishimura M, Ohtsuka K, Iwai N, Takahashi H, Yoshimura M (1999) Regulation of brain renin-angiotensin system by benzamil-blockable sodium channels. Am J Physiol 276(5 Pt 2):R1416–R1424
Nishimura M, Ohtsuka K, Nanbu A, Takahashi H, Yoshimura M (1998) Benzamil blockade of brain Na+ channels averts Na+-induced hypertension in rats. Am J Physiol 274(3 Pt 2):R635–R644
Okada Y (1997) Volume-expansion sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. Am J Physiol 273:C755–C789
Ordaz B, Vaca L, Franco R, Pasantes-Morales H (2004) Volume changes and whole cell membrane currents activated during gradual osmolarity decrease in C6 glioma cells: contribution of two types of K+ channels. Am J Physiol Cell Physiol 286:C1399–C1409
Pasantes-Morales H, Lezama RA, Ramos-Mandujanu G, Tuz KL (2006) Mechanisms of cell volume regulation in hypo-osmolality. Am J Med 119(7a):S4–S11
Pasantes-Morales H, Morales Mulia S (2000) Influence of calcium on regulatory volume decrease: role of potassium channels. Nephron 86:414–427
Sterns RH, Silver SM (2006) Brain volume regulation in response to hypo-osmolality and its correction. Am J Med 119(7A):S12–S16
Sulyok E, Pal J, Vajda Z, Steier R, Doczi T (2009) Benzamil prevents brain water accumulation in hyponatremic rats. Acta Neurochir (Wien) 151:1121–1125
Taruno A, Niisato N, Marunaka Y (2007) Hypotonicity stimulates renal epithelial sodium transport by activating JNK via receptor tyrosine kinase. Am J Physiol 293:F128–F138
Upadhyay A, Jaber BL, Madis NE (2006) Incidence and prevalence of hyponatremia. Am J Med 119(7A):S30–S35
Vajda Z, Pedersen M, Dóczi T, Sulyok E, Stødkilde-Jørgensen H, Frøkiaer J, Nielsen S (2001) Effects of centrally administered arginine vasopressin and atrial natriuretic peptide on the development of brain edema in hyponatremic rats. Neurosurgery 49:697–704
Vajda Z, Pedersen M, Füchtbauer EM, Wertz K, Stødkilde-Jørgensen H, Sulyok E, Dóczi T, Neely JD, Agre P, Frøkiaer J, Nielsen S (2002) Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenc mice. Proc Natl Acad Sci USA 99:13131–13136
Vajda Z, Promeneur D, Dóczi T, Sulyok E, Frøkiaer J, Ottersen OP, Nielsen S (2000) Increased aquaporin-4 immunoreactivity in rat brain in response to systemic hyponatremia. Biochem Biophys Res Comm 270:495–503
Wang H-W, Amin MS, El-Shahat E, Huang BS, Tuana BS, Leenen FHH (2010) Effects of central sodium on epithelial sodium channels in rat brain. Am J Physiol Regul Integr Comp Physiol 299:R222–R233
Wills NK, Millinoff LP, Crowe WE (1991) Na+ channel activity in cultured renal (A6) epithelium: regulation by solution osmolarity. J Membr Biol 121:79–90
Yool AJ, Brown EA, Flynn GA (2010) Roles for novel pharmacological blockers of aquaporins in the treatment of brain oedema and cancer. Clin Exp Pharmacol Physiol 37:403–409
Acknowledgements
This study was supported by the following grants: Marie Curie Research Training Networks Aquaglyceroporins (MRTN-CT-2006-035995); Hungarian Science Founds (OTKA PD 72240); “Save what can be saved”—Applied neurological research using high-field magnetic resonance imaging HU0114; “Visible pain”—New fMRI methods in pain research and treatment. S.A. and J.J. were supported by Bolyai fellowship of the Hungarian Academy of Science.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The authors present further data supporting the use of benzamil in the treatment of cerebral edema. Their data offer encouraging evidence that the intraventricular administration of benzamil halts the evolution of cerebral edema that would otherwise accompany acute hyponatremia. While the data generated here are something of a reiteration of past studies demonstrating reductions in water content post induction of hyponatremia and benzamil infusion in rodents, they have expanded their studies to note that the effects of ENac modulation via benzamil may be tracked by magnetic resonance imaging. There remains much work to be done before such a drug could be considered clinically viable, but these data make significant steps towards that end.
Markus Bookland,
Christopher M. Loftus
Philadelphia, USA
Rights and permissions
About this article
Cite this article
Steier, R., Aradi, M., Pál, J. et al. The influence of benzamil hydrochloride on the evolution of hyponatremic brain edema as assessed by in vivo MRI study in rats. Acta Neurochir 153, 2091–2097 (2011). https://doi.org/10.1007/s00701-011-0996-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-0996-3