Abstract
Aims
Improvement in closed-loop insulin delivery systems could result from customization of settings to individual needs and remote monitoring. This pilot home study evaluated the efficacy and relevance of this approach.
Methods
A bicentric clinical trial was conducted for 3 weeks, using an MPC-based algorithm (Diabeloop Artificial Pancreas system) featuring five settings designed to modulate the reactivity of regulation. Remote monitoring was ensured by expert nurses with a web platform generating automatic Secured Information Messages (SIMs) and with a structured procedure. Endpoints were glucose metrics and description of impact of monitoring on regulation parameters.
Results
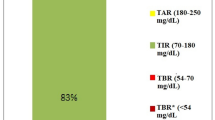
Eight patients with type 1 diabetes (six men, age 41.8 ± 11.4 years, HbA1c 7.7 ± 1.0%) were included. Time spent in the 70–180 mg/dl range was 70.2% [67.5; 76.9]. Time in hypoglycemia < 70 mg/dl was 2.9% [2.1; 3.4]. Eleven SIMs led to phone intervention. Original default settings were modified in all patients by the intervention of the nurses.
Conclusion
This pilot trial suggests that the Diabeloop closed-loop system could be efficient regarding metabolic outcomes, whereas its telemedical monitoring feature could contribute to enhanced efficacy and safety.
This study is registered at ClinicalTrials.gov with trial registration number NCT02987556.

Similar content being viewed by others
References
Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA (2017) Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 5:501–512
Pettus J, Von Herrath M (2018) The shifting paradigm of a “cure” for type 1 diabetes: is technology replacing immune-based therapies? Acta Diabetol 55:117–120
Maahs DM, Buckingham BA, Castle JR et al (2016) Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care 39:1175–1179
Hovorka R, Canonico V, Chassin LJ et al (2004) Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas 25:905–920
Bally L, Thabit H, Kojzar H et al (2017) Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol 5:261–270
El-Khatib FH, Balliro C, Hillard MA et al (2017) Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet 389:369–380
Haidar A, Messier V, Legault L, Ladouceur M, Rabasa-Lhoret R (2017) Outpatient 60-hour day-and-night glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or sensor-augmented pump therapy in adults with type 1 diabetes: an open-label, randomised, crossover, controlled trial. Diabetes Obes Metab 19:713–720
Kropff J, Del Favero S, Place J et al (2015) 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol 3:939–947
Nimri R, Muller I, Atlas E et al (2014) MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care 37:3025–3032
Thabit H, Tauschmann M, Allen JM et al (2015) Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 373:2129–2140
Castle JR, DeVries JH, Kovatchev B (2017) Future of automated insulin delivery systems. Diabetes Technol Ther 19:S67–S72
Keith-Hynes P, Guerlain S, Mize B et al (2013) DiAs user interface: a patient-centric interface for mobile artificial pancreas systems. J Diabetes Sci Technol 7:1416–1426
Place J, Robert A, Ben Brahim N et al (2013) DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Technol 7:1427–1435
Anderson SM, Raghinaru D, Pinsker JE et al (2016) Multinational home use of closed-loop control is safe and effective. Diabetes Care 39:1143–1150
Breton MD, Chernavvsky DR, Forlenza GP et al (2017) Closed loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the Artificial Pancreas Ski study. Diabetes Care. https://doi.org/10.2337/dc17-0883
DeSalvo DJ, Keith-Hynes P, Peyser T et al (2014) Remote glucose monitoring in cAMP setting reduces the risk of prolonged nocturnal hypoglycemia. Diabetes Technol Ther 16:1–7
Garg SK, Weinzimer SA, Tamborlane WV et al (2017) Glucose Outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 19:155–163
Bally L, Thabit H, Tauschmann M et al (2017) Assessing the effectiveness of a 3-month day-and-night home closed-loop control combined with pump suspend feature compared with sensor-augmented pump therapy in youths and adults with suboptimally controlled type 1 diabetes: a randomised parallel study protocol. BMJ Open 7:e016738
Gildersleeve R, Riggs SL, Cherñavvsky DR, Breton MD, DeBoer MD (2017) Improving the safety and functionality of an artificial pancreas system for use in younger children: input from parents and physicians. Diabetes Technol Ther. https://doi.org/10.1089/dia.2017.0150
Messori M, Kropff J, Del Favero S et al (2017) Individually adaptive artificial pancreas in subjects with type 1 diabetes: a one-month proof-of-concept trial in free-living conditions. Diabetes Technol Ther. https://doi.org/10.1089/dia.2016.0463
Wang Y, Zhang J, Zeng F et al (2017) “Learning” can improve the blood glucose control performance for type 1 diabetes mellitus. Diabetes Technol Ther 19:41–48
Dassau E, Pinsker JE, Kudva YC et al (2017) 12-Week 24/7 ambulatory artificial pancreas with weekly adaptation of insulin delivery settings: effect on hemoglobin A1c and hypoglycemia. Diabetes Care. https://doi.org/10.2337/dc17-1188
Charpentier G, Benhamou PY, Dardari D et al (2011) The DIABEO software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 study). Diabetes Care 34:533–539
Journal Officiel de la République Française. https://www.legifrance.gouv.fr/eli/arrete/2017/4/25/AFSH1711560A/jo/texte. Accessed 1 Mar 2018
Farrington C (2017) Hacking diabetes: dIY artificial pancreas systems. Lancet Diabetes Endocrinol 5:332
O’Keeffe DT, Maraka S, Basu A, Keith-Hynes P, Kudva YC (2015) Cybersecurity in artificial pancreas experiments. Diabetes Technol Ther 17:664–666
Acknowledgements
CERITD (Centre d’Études et de Recherches pour l’Intensification du Traitement du Diabète) is a nonprofit clinical translational research center located in Corbeil hospital. CERITD as the sole sponsor was fully involved in the design and coordination of the study. Part of the investigations was conducted within the Grenoble Clinical Research Centre (CIC-INSERM, Grenoble University Hospital) under the supervision of Pr Jean Luc Cracowski. The authors would like to thank Ilham Xhaard, Caroline Peschard, Dina Ingrao Lecante, Laurent Orlando, Delphine Coto, Anne Cecile Gully, Steeve Mounier, Marie Helene Petit from CERITD, Adeline Paris, Claire Cracowski, Enkelejda Hodaj, Justine Cristante, Manon Jalbert from Grenoble University Hospital, for involvement in the management of the study, and all engineers from DIABELOOP S.A. and from CEA-LETI. Statistical analysis was performed by Didier Not (www.rcts.fr). Financial support of the study was provided by a contract grant from AVIESAN and by CERITD.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare the following disclosures: Erik Huneker is CEO of Diabeloop S.A. Guillaume Charpentier is chairman of Diabeloop S.A. Erik Huneker, Guillaume Charpentier and Sylvia Franc are shareholders in Diabeloop S.A. The other authors (Pierre Y. Benhamou, Maeva Doron, and all other members of the Diabeloop consortium) declare no conflict of interest.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients prior to their inclusion in the study.
Additional information
Managed by Antonio Secchi.
Appendix
Appendix
The Diabeloop Consortium:
Guillaume Charpentier (CERITD, Evry, France).
Pierre Yves Benhamou (Department of Diabelology, Grenoble Alpes University, Grenoble, France).
Sophie Borot (Department of Endocrinology, Metabolism, Diabetology and Nutrition, University Hospital Jean Minjoz, Besançon, France).
Lucy Chaillous (Hospital Laennec, University Hospital of Nantes, Saint-Herblain, France).
Brigitte Delemer (Endocrinology Diabetology, Reims University Hospital, Reims, France).
Maeva Doron (CEA LETI MINATEC, Grenoble, France).
Anne Faret (Department of Endocrinology, Diabetes and Nutrition, University Hospital, Montpellier, France).
Sylvia Franc (CERITD, Evry, France).
Bruno Guerci (Endocrinology-Diabetes Care Unit, University of Lorraine, Vandoeuvre Lès Nancy, France).
Erik Huneker (DIABELOOP SA, Paris, France).
Pierre Jallon (CEA LETI MINATEC, Grenoble, France).
Nathalie Jeandidier (Department of Endocrinology, Diabetes and Nutrition, University Hospital of Strasbourg, Strasbourg, France).
Michael Joubert (Department of Endocrinology, University of Caen Côte de Nacre Regional Hospital Center, Caen, France).
Helene Hanaire (Department of Diabetology, Metabolic Diseases and Nutrition, University Hospital of Toulouse, University of Toulouse, Toulouse, France).
Sandrine Lablanche (Department of Diabelology, Grenoble Alpes University, Grenoble, France).
Céline Lukas (Endocrinology Diabetology, Reims University Hospital, Reims, France).
Vincent Melki (Department of Diabetology, Metabolic Diseases and Nutrition, University Hospital of Toulouse, University of Toulouse, Toulouse, France).
Laurent Meyer (Department of Endocrinology, Diabetes and Nutrition, University Hospital of Strasbourg, Strasbourg, France).
Alfred Penfornis (Department of Diabetes, Sud-Francilien Hospital, University Paris-Sud, Orsay, Corbeil-Essonnes, France).
Eric Renard (Department of Endocrinology, Diabetes and Nutrition, University Hospital, Montpellier, France).
Yves Reznik (Department of Endocrinology, University of Caen Côte de Nacre Regional Hospital Center, Caen, France).
Pauline Schaepelynk (Department of Nutrition-Endocrinology-Metabolic Disorders, Marseille University Hospital, Sainte Marguerite Hospital, Marseille, France).
Chantal Simon (Hospices Civils de Lyon, Department of Endocrinology Diabetology Nutrition, Lyon, France).
Charles Thivolet (Hospices Civils de Lyon, Department of Endocrinology Diabetology Nutrition, Lyon, France).
Rights and permissions
About this article
Cite this article
Benhamou, P.Y., Huneker, E., Franc, S. et al. Customization of home closed-loop insulin delivery in adult patients with type 1 diabetes, assisted with structured remote monitoring: the pilot WP7 Diabeloop study. Acta Diabetol 55, 549–556 (2018). https://doi.org/10.1007/s00592-018-1123-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1123-1