Abstract
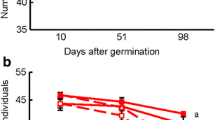
The biomass–density relationship (whereby the biomass of individual plants decreases as plant density increases) has generally been explained by competition for resources. Arbuscular mycorrhizal fungi (AMF) are able to affect plant interactions by mediating resource utilization, but whether this AMF-mediated interaction will change the biomass–density relationship is unclear. We conducted an experiment to test the hypothesis that AMF will shift the biomass–density relationship by affecting intraspecific competition. Four population densities (10, 100, 1,000, or 10,000 seedlings per square meter) of Medicago sativa L. were planted in field plots. Water application (1,435 or 327.7 mm/year) simulated precipitation in wet areas (sufficient water) and arid areas (insufficient water). The fungicide benomyl was applied to suppress AMF in some plots (“low-AMF” treatment) and not in others (“high-AMF” treatment). The effect of the AMF treatment on the biomass–density relationship depended on water conditions. High AMF enhanced the decrease of individual biomass with increasing density (the biomass–density line had a steeper slope) when water was sufficient but not when water was insufficient. AMF treatment did not affect plant survival rate or population size but did affect absolute competition intensity (ACI). When water was sufficient, ACI was significantly higher in the high-AMF treatment than in the low-AMF treatment, but ACI was unaffected by AMF treatment when water was insufficient. Our results suggest that AMF status did not impact survival rate and population size but did shift the biomass–density relationship via effects on intraspecific competition. This effect of AMF on the biomass–density relationship depended on the availability of water.







Similar content being viewed by others
References
Allen EB, Allen ME (1986) Water relations of xeric grasses in the field: interactions of mycorrhizas and competition. New Phytol 104:559–571
Allsopp N, Stock WD (1992) Density dependent interactions between VA mycorrhizal fungi and even-aged seedlings of two perennial Fabaceae species. Oecologia 91:281–287
Ayres RL, Gange AC, Aplin DM (2006) Interactions between arbuscular mycorrhizal fungi and intra-specific competition affect size, and size inequality, of Plantago lanceolata L. J Ecol 94:285–294
Callaway RM, Thelen GC, Barth S, Ransey P, Gannon JE (2004) Soil fungi alter interactions between the invader Centaurea maculosa and North American natives. Ecology 85:1062–1071
Campo RJ, Araujo RS, Hungria M (2009) Nitrogen fixation with the soybean crop in Brazil: compatibility between seed treatment with fungicide and bradyrhizobial inoculants. Symbiosis 48:154–163
Chu CJ, Maestre FT, Xiao S, Weiner J, Wang YS, Duan ZH, Wang G (2008) Balance between facilitation and resource competition determines biomass–density relationships in plant populations. Ecol Lett 11:1189–1197
Daleo P, Alberti J, Canepuccia A, Escapa M, Fanjul E, Silliman BR, Bertness MK, Iribarne O (2008) Mycorrhizal fungi determine salt-marsh plant zonation depending on nutrient supply. J Ecol 96:431–437
Deng JM, Wang GX, Morris EC, Wei XP, Li DX, Chen BM, Zhao CM, Liu J, Wang Y (2006) Plant mass–density relationship along a moisture gradient in north-west China. J Ecol 94:953–958
Eissenstat DM, Newman EI (1990) Seedling establishment near large plants: effects of vesicular–arbuscular mycorrhizas on the intensity of plant competition. Funct Ecol 4:95–99
Enquist BJ, Brown JH, West GB (1998) Allometric scaling of plant energetics and population density. Nature 395:163–165
Eriksson Å (2001) Arbuscular mycorrhiza in relation to management history, soil nutrients and plant species diversity. Plant Ecol 155:129–137
Facelli E, Facelli JM (2002) Soil phosphorus heterogeneity and mycorrhizal symbiosis regulate plant intra-specific competition and size distribution. Oecologia 133:54–61
Facelli E, Facelli JM, Smith SE, Mclaughlin MJ (1999) Interactive effects of arbuscular mycorrhizal symbiosis, intraspecific competition and resource availability on Trifolium subterraneum cv. Mt. Barker. New Phytol 141:535–547
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular–arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gurevitch J, Wilson P, Stone JL, Teese P, Stoutenburgh RJ (1990) Competition among old-field perennials at different levels of soil fertility and available space. J Ecol 78:727–744
Hartnett DC, Wilson GWT (1999) Mycorrhizae influence plant community structure and diversity in tall grass prairie. Ecology 80:1187–1195
Hartnett DC, Hetrick BAD, Wilson GWT, Gibson DJ (1993) Mycorrhizal influence of intra- and inter-specific neighbor interactions among co-occurring prairie grasses. J Ecol 81:787–795
Hashem FM, Saleh SA, van Berkum P, Voll M (1997) Survival of Bradyrhizobium sp. (Arachis) on fungicide-treated peanut seed in relationship to plant growth and yield. World J Microb Biotechnol l13:335–340
Helgason T, Merryweather JW, Young JPW, Fitter AH (2007) Specificity and resilience in the arbuscular mycorrhizal fungi of a natural woodland community. J Ecol 95:623–630
Hetrick BAD, Kitt DG, Wilson GWT (1986) The influence of phosphorus fertilization, drought, fungus species and non-sterile soil on mycorrhizal growth responses in tallgrass prairie plants. Can J Bot 64:1199–1203
Hossain AKM, Alexander M (1984a) Enhancing growth and nitrogen uptake by soybeans using pesticides. Plant Soil 81:133–141
Hossain AKM, Alexander M (1984b) Enhancing soybean rhizosphere colonization by Rhizobium japonicum. Appl Environ Microbiol 48:468–472
Koide RT (1991) Density-dependent response to mycorrhizal infection in Abutilon theophrasti Medic. Oecologia 85:389–395
Koide RT, Dickie IA (2002) Effects of mycorrhizal fungi on plant populations. Plant Soil 244:307–317
Landis FC, Gargas A, Givnish TJ (2005) The influence of arbuscular mycorrhizae and light on Wisconsin (USA) sand savanna understories 2. Plant competition. Mycorrhiza 15:555–562
Moora M, Zobel M (1996) Effect of arbuscular mycorrhiza on inter- and intra-specific competition of two grassland species. Oecologia 108:79–84
Morris EC (1999) Density-dependent mortality induced by low nutrient status of the substrate. Ann Bot 17:95–107
Morris EC (2002) Self-thinning lines differ with fertility level. Ecol Res 17:17–28
Morris EC (2003) How does fertility of the substrate affect intraspecific competition? Evidence and synthesis from self-thinning. Ecol Res 18:287–305
O’Connor PJ, Smith SE, Smith FA (2002) Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytol 154:209–218
Petraitis PS (1995) The role of growth in maintaining spatial dominance by mussels (Mytilus edulis). Ecology 76:1337–1346
Requena N, Jeffries P, Barea JM (1996) Assessment of natural mycorrhizal potential in a desertified semiarid ecosystem. Appl Environ Microbiol 62:842–847
Ronsheim ML, Anderson SE (2001) Population-level specificity in the plant–mycorrhizae association alters intraspecific interactions among neighboring plants. Oecologia 128:77–84
Scheublin TR, van Logtestijn RSP, van der Heijden MGA (2007) Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J Ecol 95:631–638
Shumway DL, Koide RT (1995) Size and reproductive inequality in mycorrhizal and nonmycorrhizal populations of Abutilon theophrasti. J Ecol 83:613–620
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic, California
Stoll P, Weiner J, Muller-Landau H, Müller E, Hara T (2002) Size symmetry of competition alters biomass–density relationships. Proc R Soc B Biol Sci 269:2191–2195
Tang QY, Feng MG (2007) DPS data processing system: experimental design, statistical analysis, and data mining. Science, Beijing
van der Heijden MGA, Wiemken A, Sanders IR (2003) Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plant. New Phytol 157:569–578
van der Putten WH, Maas PWTh, van Gulik WAM, Grinkman H (1990) Characterization of soil organisms involved in the degeneration of Ammophila arenaria. Soil Biol Biochem 22:845–852
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev 81:259–291
Weiner J, Stoll P, Muller-Landau H, Jasentuliyana A (2001) The effects of density, spatial pattern and competitive symmetry on size variation in simulated plant population. Am Nat 158:438–450
Westoby W (1984) The self-thinning rule. Adv Ecol Res 14:167–226
Yang RY (2007) The response of arbuscular mycorrhiza to environmental change and their feedbacks on host plants. Ph.D. thesis, Zhejiang University
Yoda K, Kira T, Ogawa H, Hozumi K (1963) Intraspecific competition among higher plants. XI. Self-thinning in overcrowded pure stands under cultivated and natural conditions. J Biol Osaka City Univ 14:107–129
Acknowledgements
This study was funded by the National Natural Science Foundation of China (NSFC, no. 30730020 and 30870405).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q., Xu, L., Tang, J. et al. Arbuscular mycorrhizal mediation of biomass–density relationship of Medicago sativa L. under two water conditions in a field experiment. Mycorrhiza 21, 269–277 (2011). https://doi.org/10.1007/s00572-010-0331-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-010-0331-5