Abstract
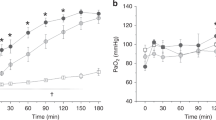
We evaluated the effects of exogenous surfactant on lung injury caused by 100% oxygen and mechanical ventilation in rabbits. Surfactant-treated rabbits (n=9) were ventilated with 100% oxygen for 36 hours and bovine surfactant was given via the trachea 12 hours after the start of mechanical ventilation. Saline-treated (n=9) rabbits were treated identically, except that they received saline without surfactant. There were no significant changes in hemodynamics, lung mechanics, or arterial oxygen tension during artificial ventilation.
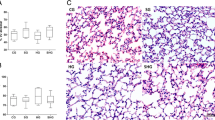
Albumin concentration in the bronchoalveolar lavage fluid (BALF) of saline-treated rabbits was slightly higher than those in surfactanttreated rabbits and significantly higher than in non-treated rabbits. C3a concentration in BALF was significantly higher in saline-treated rabbits than in surfactant-treated and non-treated rabbits. In addition, the wet-to-dry lung weight ratio was significantly lower in surfactanttreated rabbits than in saline-treated rabbits (5.06±0.10 vs. 5.67±0.14,P<0.05).
Light microscopy revealed hyaline membrane formation in saline-, treated rabbits, but fewer changes were observed in surfactant-treated rabbits. Electron microscopy revealed extensive endothelial cell destruction in saline-treated rabbits, while such changes except endothelial cell swelling were not observed in surfactant-treated rabbits.
We conclude that exogenous surfactant attenuated lung injury caused by oxygen exposure and ventilation.
Similar content being viewed by others
References
Klein J: Normobaric pulmonary oxygen toxicity, Anesth Analg 70:195–207, 1990
Clark JM, Lambertsen CJ: Pulmonary oxygen toxicity. A review. Pharmacol Rev 23:37–133, 1971
Kistler GS, Caldwell PRB, Weibel ER: Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lung. J Cell BioI 32:605–628, 1967
Obara H, Hoshino Y, Mori M, et al: Endothelium-dependent relaxation in isolated pulmonary arteries from rabbits exposed to hyperoxia. Crit Care Med 17:780–785, 1989
Obara H, Sekimoto M, Iwai S: Alterations to the bronchial and bronchiolar surfaces of adult mice after exposure to high concentrations of oxygen. Thorax 34:479–485, 1979
Crapo JD: Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol 48:721–731, 1986
Matalon S, Holm BA, Notter RH: Mitigation of pulmonary hyperoxic injury by administration of exogenous surfactant. J Appl Physiol 62:756–761, 1987
Loewen GM, Holm BA, Milanowski L, et al: Alveolar hyperoxic injury in rabbits receiving exogenous surfactant. J Appl Physiol 66:1087–1092, 1989
Crapo JD, Barry BE, Foscue HA, et al: Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis 122:123–143, 1980
Fox RB, Hoidal JR, Brown DM, et al: Pulmonary inflammation due to oxygen toxicity: Involvement of chemotactic factors and polymorphonuclear leukocytes. Am Rev Respir Dis 123:521–523, 1981
Barry BE, Crapo JD: Pattern of accumulation of platelets and neutrophils in rat lungs during exposure to 100% and 85% oxygen. Am Rev Respir Dis 132:548–555, 1985
Rinaldo JE, English D, Levine J, et al: Increased intrapulmonary retension of radiolabeled neutrophils in early oxygen toxicity. Am Rev Respir Dis 137:345–352, 1988
LeSouf PN, England SJ, Bryan AC: Total resistance of the respiratory system in preterm infants with and without an endotracheal tube. J Pediatr 104:108–111, 1984
Takayama M, Itoh S, Nagasaki T, et al: A new enzymatic method for determination of serum cholinecontaining phospholipids. Clin Chim Acta 79:93–98, 1977
de los Santos R, Seidenfeld JJ, Anzueto A, et al: One hundred percent oxygen lung injury in adult baboons. Am Rev Respir Dis 136:657–661, 1987
Davis WB, Stephen I, Rennard SI, et al: Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med 309:878–883, 1983
Matalon S, Cesar MA: Effects of 100% oxygen breathing on the capillary filtration coefficient in rabbit lungs. Microvasc Res 29:70–80, 1985
Dreyfuss D, Soler P, Basset G, et al: High inflation pressure pulmonary edema. Am Rev Respir Dis 137:1159–1164, 1988
Kolobow T, Moretti MP, Fumagalli R, et al: Severe impairment in function induced by high peak airway pressure during mechanical ventilation. Am Rev Respir Dis 135:312–315, 1987
Gross NJ, Smith DM: Impaired surfactant phospholipid metabolism in hyperoxic mouse lungs. J Appl Physiol 51:1198–1203, 1981
Holm BA, Matalon S, Finkelstein IN, et al: Type II pneumocyte changes during hyperoxic lung injury and recovery. J Appl Physiol 65:2672–2678, 1988
Kobayashi T, Nitta K, Ganzuka M, et al: Inactivation of exogenous surfactant by pulmonary edema fluid. Pediatr Res 29:353–356, 1991
Fuchimukai T, Fujiwara T, Takahashi A, et al: Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol 62:429–437, 1987
Seeger W, Stöhr G, Wolf HRD, et al: Alteration of surfactant function due to protein leakage: special interaction with fibrin monomer. J Appl Physiol 58:326–338, 1985
Holm BA, Enhorning G, Notter RH: A biophysical mechanism by which plasma proteins inhibit lung surfactant activity. Chern Phys Lipids 49:49–55, 1988
Jobe A, Ikegami M, Jacobs H, et al: Permeability of premature lamb lungs to protein and the effect of surfactant on that permeability. J Appl Physiol 55:169–176,1983
Hills BA: Water repellency induced by pulmonary surfact.ants. J Physiol 325:175–186, 1982
Hills BA: ‘De-watering’ capability of surfactants in human amniotic fluid. J Physiol 348:369–381, 1984
Fridovich I: The biology of oxygen radicals. Science 201:875–880, 1978
Halliwell B, Gutteridge JMC: Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–14, 1984
Buckley BJ, Tanswell AK, Freeman BR: Liposome-mediated augmentation of catalase in alveolar type II cells protects against H202 injury. J Appl Physiol 63:359–367, 1987
Tanswell AK, Freeman BA: Liposomeentrapped antioxidant enzymes prevent lethal O2 toxicity in the newborn rat. J Appl Physiol 63:347–352, 1987
Turrens JF, Crapo JD, Freeman BA: Protection against oxygen toxicity by intravenous injection of liposomeentrapped catalase and superoxide dismutase. J Clin Invest 73:87–95, 1984
Matalon S, Holm BA, Baker RR, et al: Antioxidant properties of surfactant replacement mixtures. Am Rev Respir Dis 137:80, 1988
Hayakawa H, Myrvik QN, Clair RWST: Pulmonary surfactant inhibits priming of rabbit alveolar macrophage. Evidence that surfactant suppress the oxidative burst of alveolar macrophage in infant rabbit. Am Rev Respir Dis 140:1390–1397, 1989
Parsons PE, Fowler AA, Hyers TM, et al: Chemotactic activity in bronchoalveolar lavage fluid from patients with adult respiratory distress syndrome. Am Rev Respir Dis 132:490–493, 1985
Merritt TA, Hallman M, Holcomb K, et al: Human surfactant treatment of severe respiratory distress syndrome: Pulmonary effluent indicators of lung inflammation. J Pediatr 108:741–748, 1986
Damiano VV, Cohen A, Tsang A, et al: A morphologic study of the influx of neutrophils into dog lung alveoli after lavage with sterile saline. Am J Pathoi 100:349–364, 1980
Kwong MS, Eagan EA, Notter RH: A double blind clinical trial of calf lung lipid for the prevention of hyaline membrane disease in extremely premature infants. Pediatrics 76:585–592, 1985
Fujiwara T, Maeta J, Chida S, et al: Artifical surfactant for treatment of hyaline-membrane disease. Lancet 1:55–59, 1980
Maeta H, Raju TNK, Vidyasagar D, et al: Effect of exogenous surfactant on the development of bronchopulmonary dysplasia in a baboon hyaline membrane disease model. Crit Care Med 18:403–409, 1990
Author information
Authors and Affiliations
About this article
Cite this article
Ikegaki, J., Mikawa, K. & Obara, H. Effects of surfactant on lung injury induced by hyperoxia and mechanical ventilation in rabbits. J Anesth 7, 66–74 (1993). https://doi.org/10.1007/s0054030070066
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s0054030070066