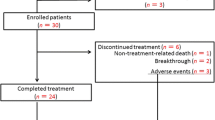
Abstract
Background
Hepatitis C virus (HCV) infection is a major comorbidity in patients receiving hemodialysis. Interferon-based antiviral therapy to eradicate HCV is less effective in patients receiving hemodialysis than patients without renal dysfunction. Recently reported combination therapy with two oral direct-acting antiviral drugs, daclatasvir and asunaprevir, both of which are metabolized in the liver and excreted into the bile ducts, reportedly showed a high rate of HCV eradication. We evaluated the safety and efficacy of this therapy in patients receiving hemodialysis.
Methods
The safety and viral responses were compared among patients infected with HCV genotype 1, between 28 patients receiving hemodialysis, and propensity score-matched 56 patients without renal dysfunction.
Results
The reduction in serum HCV RNA levels 1 day after the start of therapy was significantly larger (p = 0.0329) and the disappearance of serum HCV RNA occurred significantly earlier (p = 0.0017) in patients receiving hemodialysis than those without renal dysfunction. The rates of sustained virologic response, i.e., the eradication of HCV, were comparable between two groups; the rate of SVR12 was 100 % in patients receiving hemodialysis and 94.6 % in patients without renal dysfunction. No adverse constitutional events were observed in either of the groups. The elevation of serum alanine aminotransferase levels, a known adverse effect of these drugs, was observed in comparable rate of patients between the two groups.
Conclusions
The therapy with daclatasvir and asunaprevir has high antiviral efficacy in patients receiving hemodialysis with a comparable safety profile to patients without renal dysfunction.




Similar content being viewed by others
References
Berenguer M. Treatment of chronic hepatitis C in hemodialysis patients. Hepatology. 2008;48(5):1690–9.
Fabrizi F, Poordad FF, Martin P. Hepatitis C infection and the patient with end-stage renal disease. Hepatology. 2002;36(1):3–10.
Martin P, Fabrizi F. Hepatitis C virus and kidney disease. J Hepatol. 2008;49(4):613–24.
Liu CH, Kao JH. Treatment of hepatitis C virus infection in patients with end-stage renal disease. J Gastroenterol Hepatol. 2011;26(2):228–39.
Stehman-Breen CO, Emerson S, Gretch D, et al. Risk of death among chronic dialysis patients infected with hepatitis C virus. Am J Kidney Dis. 1998;32(4):629–34.
Pereira BJ, Natov SN, Bouthot BA, et al. Effects of hepatitis C infection and renal transplantation on survival in end-stage renal disease. Kidney Int. 1998;53(5):1374–81.
Espinosa M, Martin-Malo A, Alvarez de Lara MA, et al. Risk of death and liver cirrhosis in anti-HCV positive long-term hemodialysis patients. Nephrol Dial Transplant. 2001;16(8):1669–74.
Nakayama E, Akiba T, Marumo F, Sato C. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol. 2000;11(10):1896–902.
Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366(3):216–24.
Chayama K, Takahashi S, Toyota J, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55(3):724–8.
Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1 infection. Hepatology. 2014;59(6):2083–91.
Garimella T, Wang R, Luo WL, et al. Single-dose pharmacokinetics and safety of daclatasvir in subject with renal function impairment. Antivir Ther. 2015;20(5):535–43.
Garimella T, He B, Luo WL, et al. Asunaprevir pharmacokinetics and safety in subjects with impaired renal function. Hepatology. 2013;58(10):430A.
Ohno O, Mizokami M, Wu RR, et al. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J Clin Microbiol. 1997;35(1):201–7.
Uchida Y, Kouyama J, Naiki K, Mochida S. A novel simple assay system to quantify the percent HCV RNA levels of NS5A Y93H mutant strains and Y93 wild-type strains relative to the total HCV-RNA levels to determine the indication for antiviral therapy with NS5A inhibitors. PLoS One. 2014;9(11):e112647.
Lopes EPA, Gouveia EC, Albuquerque ACC, et al. Determination of the cut-off of serum alanine aminotransferase in patients undergoing hemodialysis, to identify biochemical activity in patients with hepatitis C viremia. J Clin Virol. 2006;35(3):298–302.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92.
Guh JY, Lai YH, Yang CY, et al. Impact of decreased serum transaminase levels on the evaluation of viral hepatitis patients. Nephron. 1995;69(4):459–65.
Yuki N, Ishida H, Inoue T, et al. Reappraisal of biochemical hepatitis C activity in hemodialysis patients. J Clin Gastroenterol. 2000;30(2):187–94.
Liu CH, Huang CF, Liu CJ, et al. Pegylated interferon-α2a with or without low-dose ribavirin for treatment-naïve patients with hepatitis C virus genotype 1 receiving hemodialysis. A randomized trial. Ann Intern Med. 2013;159(11):729–38.
Deltenre P, Moreno C, Tran A, et al. Anti-viral therapy in haemodialysed HCV patients: efficacy, tolerance and treatment strategy. Alim Pharmacol Ther. 2011;34(4):454–61.
Toyoda H, Kumada T, Kiriyama S, et al. An early viral response to standard interferon-alpha identifies resistance to combination therapy with peginterferon and ribavirin in patients infected by HCV genotype 1. J Med Virol. 2010;82(9):1537–44.
Toyoda H, Kumada T, Tada T, et al. Association between HCV amino acid substitutions and outcome of peginterferon and ribavirin combination therapy in HCV genotype 1b and high viral load. J Gastroenterol Hepatol. 2010;25(6):1072–8.
Fujiwra K, Kaneko S, Kakumu S, et al. Double filtration plasmapheresis and interferon combination therapy for chronic hepatitis C patients with genotype 1 and high viral load. Hepatol Res. 2007;37(9):701–10.
Kaneko S, Sata M, Ide T, et al. Efficacy and safety of double filtration plasmapheresis in combination with interferon therapy for chronic hepatitis C. Hepatol Res. 2010;40(11):1072–81.
Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P. Meta-analysis: interferon for the treatment of chronic hepatitis C in dialysis patients. Aliment Pharmacol Ther. 2003;18(11–12):1071–81.
Russo MW, Goldsweig CD, Jacobson IM, Brown RS Jr. Interferon monotherapy for dialysis patients with chronic hepatitis C: an analysis of the literature on efficacy and safety. Am J Gastroenterol. 2003;98(7):1610–5.
Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–8.
Ikeda K, Saitoh S, Arase Y, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long-term observation study of 1643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124–30.
Imai Y, Kawata S, Tamura S, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Ann Intern Med. 1998;129:94–9.
Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. Ann Intern Med. 1999;131:174–81.
Kasahara A, Hayashi N, Mochizuki K, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Hepatology. 1998;27:1394–402.
Ogawa E, Furusyo N, Kajiwara E, et al. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma of patients with chronic hepatitis C: a prospective multicenter study. J Hepatol. 2013;58:495–501.
van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93.
Lee MH, Yang HI, Lu SN, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–77.
Tada T, Kumada T, Toyoda H, et al. Viral eradication reduces all-cause mortality in patients with chronic hepatitis C virus infection: a propensity score analysis. Liver Int (in press).
Tanaka J, Katayama K, Matsuo J, et al. The association of hepatitis C virus infection with the prognosis of chronic hemodialysis patients: a retrospective study of 3,064 patients between 1999 and 2010. J Med Virol. 2015;87:1558–64.
Roth D, Nelson DR, Bruchefeld A, et al. Grazoprevir plus elbasvir in treatment-naïve and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 2015;386(10):1537–45.
Pockros PJ, Reddy KR, Mantry PS, et al. Safety of ombitasvir/paritaprevir/ritonavir plus dasabuvir for treating HCVGT1 infection in patients with severe renal impairment or end-stage renal disease: the RUBY-1 study. J Hepatol. 2015;62(Suppl 1):S1145A.
Acknowledgments
The authors thank Dr. Shintaro Ogawa, Department of Virology and Liver Unit, Nagoya City University Graduate School of Medical Science, for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Toyoda, H., Kumada, T., Tada, T. et al. Safety and efficacy of dual direct-acting antiviral therapy (daclatasvir and asunaprevir) for chronic hepatitis C virus genotype 1 infection in patients on hemodialysis. J Gastroenterol 51, 741–747 (2016). https://doi.org/10.1007/s00535-016-1174-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1174-4