Abstract
Background
Little is known about stopping rules of nucelos(t)ide analog (NA) treatment for chronic hepatitis B (CHB).
Methods
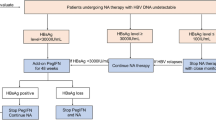
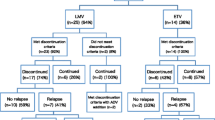
A total of 113 consecutive patients with CHB (45 HBeAg-positive and 68 HBeAg-negative CHB patients), who met the cessation criteria of NA treatment as per the Asian-Pacific Association for the Study of the Liver (APASL) guideline, were enrolled in this prospective cohort study. The primary endpoint was to evaluate virological relapse (VR) rate within 1 year, which was defined as reappearance of hepatitis B virus (HBV)–DNA > 2000 IU/mL after cessation of NA treatment. In this cohort, entecavir was used in 81 (71.7 %) and lamivudine in 32 (28.3 %) patients.
Results
Within 1 year after NA treatment, VR occurred in 26 (57.8 %) HBeAg-positive patients and in 37 (54.4 %) HBeAg-negative patients. In univariate and subsequent multivariate analysis, age > 40 years [odds ratio (OR) 10.959; 95 % confidence interval (CI) 2.211–54.320; P = 0.003) and a pre-treatment HBV DNA level >2000,000 IU/mL (OR 9.285; 95 % CI 1.545–55.795; P = 0.036) were identified as independent risk factors for VR in HBeAg-positive patients, and age > 40 years (OR 6.690; 95 % CI 1.314–34.057; P = 0.022) and an end-of-treatment HBcrAg level >3.7 log IU/mL (OR 3.751; 95 % CI 1.187–11.856; P = 0.024) were identified in HBeAg-negative patients. During follow up, neither hepatic decompensation nor hepatocellular carcinoma (HCC) occurred, and HBV DNA suppression was achieved in all patients who received antiviral re-treatment.
Conclusion
Our data suggested that the APASL stopping rule could be applied if a candidate was properly selected using individual risk factors. However, regular monitoring should be performed after cessation of NA treatment and long-term outcomes need to be evaluated further.



Similar content being viewed by others
Abbreviations
- HBV:
-
Hepatitis B virus
- CHB:
-
Chronic hepatitis B
- PEG-IFN:
-
Pegylated interferon
- NA:
-
Nucelos(t)ide analog
- HBsAg:
-
Hepatitis B surface antigen
- AASLD:
-
American Association for the Study of Liver Disease
- EASL:
-
European Association for the Study of the Liver
- APASL:
-
Asian-Pacific Association for the Study of the Liver
- LAM:
-
Lamivudine
- ETV:
-
Entecavir
- PCR:
-
Polymerase chain reaction
- VR:
-
Virological relapse
- ALT:
-
Alanine aminotransferase
- ULN:
-
Upper limit of normal
- HBcrAg:
-
HBV core-related antigen
- IP-10:
-
Interferon gamma-induced protein 10
- LS:
-
Liver stiffness
- AST:
-
Aspartate aminotransferase
- kPa:
-
Kilopascals
- IQR:
-
Interquartile range
- IQR/M:
-
IQR to median value ratio
- SD:
-
Standard deviation
- OR:
-
Odd ratio
- CI:
-
Confidence interval
- TDF:
-
Tenofovir
- cccDNA:
-
Covalently closed circular DNA
References
Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107.
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–2.
Yapali S, Talaat N, Lok AS. Management of hepatitis B: our practice and how it relates to the guidelines. Clin Gastroenterol Hepatol. 2014;12(1):16–26.
Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–31.
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–75.
EASL Clinical Practice Guidelines. Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–85.
Liaw Y-F, Kao J-H, Piratvisuth T, Chan H, Chien R-N, Liu C-J, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6(3):531–61.
Kang W, Park JY. When to stop nucleos(t)ide analogues treatment for chronic hepatitis B? Durability of antiviral response. World J Gastroenterol. 2014;20(23):7207–12.
Yeh CT, Hsu CW, Chen YC, Liaw YF. Withdrawal of lamivudine in HBeAg-positive chronic hepatitis B patients after achieving effective maintained virological suppression. J Clin Virol. 2009;45(2):114–8.
Fung J, Lai CL, Tanaka Y, Mizokami M, Yuen J, Wong DK, et al. The duration of lamivudine therapy for chronic hepatitis B: cessation vs. continuation of treatment after HBeAg seroconversion. Am J Gastroenterol. 2009;104(8):1940–6.
Dienstag JL, Schiff ER, Mitchell M, Casey DE Jr, Gitlin N, Lissoos T, et al. Extended lamivudine retreatment for chronic hepatitis B: maintenance of viral suppression after discontinuation of therapy. Hepatology. 1999;30(4):1082–7.
Dienstag JL, Cianciara J, Karayalcin S, Kowdley KV, Willems B, Plisek S, et al. Durability of serologic response after lamivudine treatment of chronic hepatitis B. Hepatology. 2003;37(4):748–55.
Song BC, Suh DJ, Lee HC, Chung YH, Lee YS. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology. 2000;32(4 Pt 1):803–6.
Lee KM, Cho SW, Kim SW, Kim HJ, Hahm KB, Kim JH. Effect of virological response on post-treatment durability of lamivudine-induced HBeAg seroconversion. J Viral Hepat. 2002;9(3):208–12.
Jung YK, Yeon JE, Lee KG, Jung ES, Kim JH, Seo YS, et al. Virologic response is not durable after adefovir discontinuation in lamivudine-resistant chronic hepatitis B patients. Korean J Hepatol. 2011;17(4):261–7.
Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143(3):629–636.e1.
Ha M, Zhang G, Diao S, Lin M, Sun L, She H, et al. A prospective clinical study in hepatitis B e antigen-negative chronic hepatitis B patients with stringent cessation criteria for adefovir. Arch Virol. 2012;157(2):285–90.
Liu F, Wang L, Li XY, Liu YD, Wang JB, Zhang ZH, et al. Poor durability of lamivudine effectiveness despite stringent cessation criteria: a prospective clinical study in hepatitis B e antigen-negative chronic hepatitis B patients. J Gastroenterol Hepatol. 2011;26(3):456–60.
Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21(9):1023–32.
Park H, Lee JM, Seo JH, Kim HS, Ahn SH, Kim DY, et al. Predictive value of HBsAg quantification for determining the clinical course of genotype C HBeAg-negative carriers. Liver Int. 2012;32(5):796–802.
Kim MN, Lee CK, Ahn SH, Lee S, Kim SU, Kim DY, et al. Maintaining remission in lamivudine-resistant patients with a virological response to adefovir add-on lamivudine after stopping lamivudine therapy. Liver Int. 2014;34(10):1543–9.
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–13.
Lee HW, Lee HJ, Hwang JS, Sohn JH, Jang JY, Han KJ, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology. 2010;51(2):415–21.
Reijnders JG, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology. 2010;139(2):491–8.
Liang Y, Jiang J, Su M, Liu Z, Guo W, Huang X, et al. Predictors of relapse in chronic hepatitis B after discontinuation of anti-viral therapy. Aliment Pharmacol Ther. 2011;34(3):344–52.
Wong DK, Yuen MF, Ngai VW, Fung J, Lai CL. One-year entecavir or lamivudine therapy results in reduction of hepatitis B virus intrahepatic covalently closed circular DNA levels. Antivir Ther. 2006;11(7):909–16.
Mealing S, Ghement I, Hawkins N, Scott DA, Lescrauwaet B, Watt M, et al. The importance of baseline viral load when assessing relative efficacy in treatment-naive HBeAg-positive chronic hepatitis B: a systematic review and network meta-analysis. Syst Rev. 2014;3:21.
Chan HL, Wang H, Niu J, Chim AM, Sung JJ. Two-year lamivudine treatment for hepatitis B e antigen-negative chronic hepatitis B: a double-blind, placebo-controlled trial. Antivir Ther. 2007;12(3):345–53.
Jeng WJ, Sheen IS, Chen YC, Hsu CW, Chien RN, Chu CM, et al. Off-therapy durability of response to entecavir therapy in hepatitis B e antigen-negative chronic hepatitis B patients. Hepatology. 2013;58(6):1888–96.
Seto WK, Hui AJ, Wong VW, Wong GL, Liu KS, Lai CL, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut. 2015;64(4):667–72.
Chan HL, Wong GL, Chim AM, Chan HY, Chu SH, Wong VW. Prediction of off-treatment response to lamivudine by serum hepatitis B surface antigen quantification in hepatitis B e antigen-negative patients. Antivir Ther. 2011;16(8):1249–57.
Jaroszewicz J, Ho H, Markova A, Deterding K, Wursthorn K, Schulz S, et al. Hepatitis B surface antigen (HBsAg) decrease and serum interferon-inducible protein-10 levels as predictive markers for HBsAg loss during treatment with nucleoside/nucleotide analogues. Antivir Ther. 2011;16(6):915–24.
Sonneveld MJ, Arends P, Boonstra A, Hansen BE, Janssen HL. Serum levels of interferon-gamma-inducible protein 10 and response to peginterferon therapy in HBeAg-positive chronic hepatitis B. J Hepatol. 2013;58(5):898–903.
Park Y, Hong DJ, Shin S, Cho Y, Kim HS. Performance evaluation of new automated hepatitis B viral markers in the clinical laboratory: two quantitative hepatitis B surface antigen assays and an HBV core-related antigen assay. Am J Clin Pathol. 2012;137(5):770–7.
Rokuhara A, Tanaka E, Matsumoto A, Kimura T, Yamaura T, Orii K, et al. Clinical evaluation of a new enzyme immunoassay for hepatitis B virus core-related antigen; a marker distinct from viral DNA for monitoring lamivudine treatment. J Viral Hepat. 2003;10(4):324–30.
Tanaka E, Matsumoto A, Yoshizawa K, Maki N. Hepatitis B core-related antigen assay is useful for monitoring the antiviral effects of nucleoside analogue therapy. Intervirology. 2008;51(Suppl 1):3–6.
Shinkai N, Tanaka Y, Orito E, Ito K, Ohno T, Hirashima N, et al. Measurement of hepatitis B virus core-related antigen as predicting factor for relapse after cessation of lamivudine therapy for chronic hepatitis B virus infection. Hepatol Res. 2006;36(4):272–6.
Matsumoto A, Tanaka E, Suzuki Y, Kobayashi M, Tanaka Y, Shinkai N, et al. Combination of hepatitis B viral antigens and DNA for prediction of relapse after discontinuation of nucleos(t)ide analogs in patients with chronic hepatitis B. Hepatol Res. 2012;42(2):139–49.
Byun KS, Kwon OS, Kim JH, Yim HJ, Chang YJ, Kim JY, et al. Factors related to post-treatment relapse in chronic hepatitis B patients who lost HBeAg after lamivudine therapy. J Gastroenterol Hepatol. 2005;20(12):1838–42.
Acknowledgments
The authors are grateful to Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the figures.
Grant support
This study was supported by following Grants; (a) a fund of the HBV cohort study from the Korea Centers for Disease Control and Prevention (4800-4845-300-260, 2015-ER5101-00), (b) a faculty research Grant of Yonsei University College of Medicine (No. 6-2012-0008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Kyu Sik Jung and Jun Yong Park have equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Cumulative relapse rate after cessation of antiviral therapy in HBeAg-positive patients (A) and HBeAg-negative patients (B). The 1-year cumulative relapse rate is not significantly different between the two groups (P > 0.05, log-rank test;
TIFF 8275 kb)
Rights and permissions
About this article
Cite this article
Jung, K.S., Park, J.Y., Chon, Y.E. et al. Clinical outcomes and predictors for relapse after cessation of oral antiviral treatment in chronic hepatitis B patients. J Gastroenterol 51, 830–839 (2016). https://doi.org/10.1007/s00535-015-1153-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1153-1