Abstract
Purpose
Rituximab is a chimeric monoclonal antibody approved to treat B cell non-Hodgkin’s lymphoma (NHL). Infusion reactions among NHL patients are common during the first exposure but decrease with subsequent infusions. We sought to assess the safety and feasibility of a rituximab rapid infusion protocol in the outpatient treatment area of a comprehensive cancer center.
Patients and methods
Patients with indolent and intermediate B cell NHL were invited to enroll in this prospective, single-institution study if they had received the first dose of rituximab according to the manufacturer-labeled standard titration schedule without grade >2 infusion reaction. The subsequent infusion proceeded without the use of steroid premedication at 100 mg/h administered over 15 min, with the remaining dose given over 45 min. Time savings between rapid infusion and standard titration were calculated.
Results
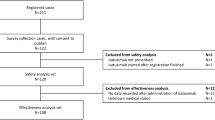
Fifty patients received 60-min rituximab infusions during the second drug administration. No infusion-related reactions of any grade were observed with the rapid infusion protocol (0 %, one-sided 97.5 % CI 0–7.1 %). The mean time for the rapid rituximab infusion was 62.4 min (95 % CI 61.2–63.6). When compared to the standard second dose infusion recommendation, a mean time of 94.2 min (95 % 90–98.4) was saved with rapid infusion. Nursing surveys demonstrated 100 % satisfaction with the rapid infusion protocol.
Conclusions
Subsequent rituximab infusions can be safely administered over 60 min and without steroid premedication in an experienced outpatient infusion center when patients are appropriately screened. The faster infusions can reduce resource utilization and increase nursing satisfaction.
Trial Registration
NCT01206777

Similar content being viewed by others
References
Rituxan® (2013) [package insert]. Genentech, Inc, South San Francisco
Rituxan® (2000) [package insert]. Hoffmann-La Roche Ltd, Mississauga
Common terminology criteria for adverse events (CTCAE): General disorders and administration site conditions. Version 4.02. September 10, 2009
Aurran-Schleinitz T, Gravis G, Vittot M et al (2005) One hour rituximab infusion is safe and improves patient care and outpatient unit management. Blood 106:4759
Sehn LH, Donaldson J, Filewich A et al (2007) Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood 109(10):4171–4173
Chiang J, Chan A, Shih V et al (2010) A prospective study to evaluate the feasibility and economic benefits of rapid infusion rituximab at an Asian cancer center. Int J Hematol 91(5):826–30
Middleton HJ, Mollee P, Bird R et al (2005) Accelerated delivery of rituximab is safe on an out-patient basis. Blood 106:4777
Siano M, Lerch E, Negretti L (2008) A phase I-II study to determine the maximum tolerated infusion rate of rituximab with special emphasis on monitoring the effect of rituximab on cardiac function. Clin Cancer Res 14(23):7935–79359
Ghielmini M, Negretti L, Lerch E et al (2005) Infusion speed-escalation trial to give full-dose rituximab in One hour without steroids Pre-medication. Blood 106:2451
Lang DSP, Keefe DMK, Schultz T et al (2013) Predictors of acute adverse events from rapid rituximab infusion. Support Care Cancer 21(8):2315–20
Tuthill M, Crook T, Corbet T et al (2009) Rapid infusion of rituximab over 60 min. Eur J Haematol 82(4):322–5
Atay S, Barista I, Gundogdu F et al (2012) Rapid-infusion rituximab in lymphoma treatment: 2-year experience in a single institution. J Oncol Pract 8(3):141–143
Dakhil, Hermann R, Schreeder MT, et al (2014) Phase III safety study of rituximab administered as a 90-minute infusion in patients with previously untreated diffuse large B-cell and follicular lymphoma. Leuk Lymphoma 55(10):2335–2340
Kallen M, Terrell J, Lewis-Patterson P et al (2012) Improving wait times for chemotherapy in an outpatient clinic at a comprehensive cancer center. J Oncol Pract 8(1):e1–e7
Griffith N, Allen C, Pultz A et al (2008) The impact of a long-acting erythropoiesis stimulating protein on patient throughput in a hospital-based ambulatory oncology clinic. Hosp Pharm 43:388–395
Swan J, Zaghloul H, Cox J et al (2014) Use of a pharmacy protocol to convert standard rituximab infusions to rapid infusion shorten outpatient infusion clinic visits. Pharmacotherapy 34(7):686–694
Conflict of interests
Emily Dotson, Brooke Crawford, and Gary Phillips have no relevant conflicts of interest. Jeffrey Jones received research funding from Genentech and consulting/advisory roles with Genentech.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dotson, E., Crawford, B., Phillips, G. et al. Sixty-minute infusion rituximab protocol allows for safe and efficient workflow. Support Care Cancer 24, 1125–1129 (2016). https://doi.org/10.1007/s00520-015-2869-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2869-4