Abstract
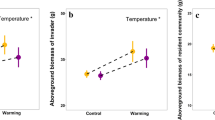
Neighbor interactions are likely to play an important role in subarctic plant communities. We conducted experiments in Interior Alaska in which we crossed species removal with greenhouse warming manipulations. We examined changes in community biomass, and in plant survival and growth of individual species in response to experimental warming and to: (1) removal of whole species versus an equivalent amount of biomass across many species, and (2) removal of subdominant (locally common) versus minor (locally uncommon) plants. Community biomass indicated compensation in growth after removal of minor species and after biomass removal without elimination of entire species, but under-compensation after removal of subdominants. Growth and survival of individual species showed facilitation between some species. Warming increased growth of dominant vascular plants, but at the same time reduced survival, and these impacts were greater for larger, more mesic species than for the smaller species associated with drier habitats. Growth of mosses was reduced by the warming. Removal effects did not differ between warming and ambient conditions. The results indicate that common species are able to reduce resources for others (competitive effect) and increase their growth after neighbor removal, whereas locally uncommon species are not able to respond rapidly to increased resources made available by neighbor removal. Therefore, the impact of the presence of common species on locally uncommon species was facilitative overall, but not vice versa. The balance between disturbances such as changes in temperature and species losses from the community will likely be crucial in determining shifts in subsequent community composition.






Similar content being viewed by others
References
Aarssen LW (1983) Ecological combining ability and competitive combining ability in plants—toward a general evolutionary theory of coexistence in systems of competition. Am Nat 122:707–731
Aksenova AA, Onipchenko VG, Blinnikov MS (1998) Plant interactions in alpine tundra: 13 years of experimental removal of dominant species. Ecoscience 5:258–270
Anderson LE, Crum HA, Buck WR (1990) List of the mosses of North-America north of Mexico. Bryologist 93:448–499
Arft AM et al (1999) Response patterns of tundra plant species to experimental warming: a meta-analysis of the International Tundra Experiment. Ecol Monogr 69:491–511
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Bertness MD, Hacker S (1994) Physical stress and positive associations among marsh plants. Am Nat 144:363–372
Bret-Harte MS et al (2004) Plant and soil responses to neighbour removal and fertilization in Alaskan tussock tundra. J Ecol 92:635–647
Brooker RW et al (2008) Facilitation in plant communities: the past, the present, and the future. J Ecol 96:18–34
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349
Callaway RM (1998) Are positive interactions species-specific? Oikos 82:202–207
Callaway RM et al (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Carlsson BA, Callaghan TV (1991) Positive plant interactions in tundra vegetation and the importance of shelter. J Ecol 79:973–983
Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA (1995) Responses of arctic tundra to experimental and observed changes in climate. Ecology 76:694–711
Choler P, Michalet R, Callaway RM (2001) Facilitation and competition on gradients in alpine plant communities. Ecology 82:3295–3308
Clymo RS, Hayward PM (1982) The ecology of Sphagnum. In: Smith AJE (ed) Bryophyte ecology. Chapman and Hall, London, pp 229–290
Del Moral R (1983) Competition as a control mechanism in subalpine meadows. Am J Bot 70:232–245
Diaz S et al (2000) Practical guidelines for removal experiments. In: GCTE/LTER Launch Workshop, GCTE network of removal experiments on the role of biodiversity in ecosystem functioning, pp1–8
Gerdol R, Brancaleoni L, Marchesini R, Bragazza L (2002) Nutrient and carbon relations in subalpine dwarf shrubs after neighbour removal or fertilization in northern Italy. Oecologia 130:476–483
Goldberg DE, Rajaniemi T, Gurevitch J, Stewart-Oaten A (1999) Empirical approaches to quantifying interaction intensity: competition and facilitation along productivity gradients. Ecology 80:1118–1131
Grace JB (1995) On the measurement of plant competition intensity. Ecology 76:305–308
Hobbie SE, Shevtsova A, Chapin FS (1999) Plant responses to species removal and experimental warming in Alaskan tussock tundra. Oikos 84:417–434
Hollister RD, Webber PJ, Bay C (2005) Plant response to temperature in Northern Alaska: Implications for predicting vegetation change. Ecology 86:1562–1570
Hultén E (1968) Flora of Alaska and neighboring territories: a manual of the vascular plants. Arctic Inst of North America, Washigton, DC
Jonasson S (1992) Plant responses to fertilization and species removal in tundra related to community structure and clonality. Oikos 63:420–429
Kitzberger T, Steinaker DF, Veblen TT (2000) Effects of climatic variability on facilitation of tree establishment in northern Patagonia. Ecology 81:1914–1924
Körner C (2003) Alpine plant life, 2nd edn. Springer, Berlin
Krummenacher B, Budmider K, Mihajlovic D, Blank B (1998) Periglaziale Prozesse und Formen im Furggentälti, Gemmipass. Mitt Eidgenössisch Inst Schnee- und Lawinenforsch Davos 56:1–245
Kudo G, Nordenhall U, Molau U (1999) Effects of snowmelt timing on leaf traits, leaf production, and shoot growth of alpine plants: comparisons along a snowmelt gradient in northern Sweden. Ecoscience 6:439–450
Michalet R et al (2006) Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol Lett 9:767–773
Moen J (1993) Positive versus negative interactions in a high alpine block field—germination of Oxyria digyna seeds in a Ranunculus glacialis community. Arctic Alp Res 25:201–206
Molau U, Molgaard P (1996) ITEX manual. Danish Polar Center, Copenhagen
Morgan JW (2006) Bryophyte mats inhibit germination of non-native species in burnt temperate native grassland remnants. Biol Invasions 8:159–168
Mulder CPH, Ruess RW (1998) Effects of herbivory on arrowgrass: interactions between geese, neighboring plants, and abiotic factors. Ecol Monogr 68:275–293
Mulder CPH, Bazeley-White E, Dimitrakopoulos PG, Hector A, Scherer-Lorenzen M, Schmid B (2004) Species evenness and productivity in experimental plant communities. Oikos 107:50–63
Nilsson MC, Wardle DA (2005) Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Frontiers Ecol 3:421–428
Okland RH, Okland T (1996) Population biology of the clonal moss Hylocomium splendens in Norwegian boreal spruce forests. II. Effects of density. J Ecol 84:63–69
Oksanen L, Sammul M, Magi M (2006) On the indices of plant-plant competition and their pitfalls. Oikos 112:149–155
Olofsson J (2004) Positive and negative plant-plant interactions in two contrasting arctic-alpine plant communities. Arctic Antarct Alp Res 36:464–467
Olofsson J, Moen J, Oksanen L (1999) On the balance between positive and negative plant interactions in harsh environments. Oikos 86:539–543
Rydgren K, Hestmark G, Okland RH (1998) Revegetation following experimental disturbance in a boreal old-growth Picea abies forest. J Veg Sci 9:763–776
Sammul M, Kull K, Oksanen L, Veromann P (2000) Competition intensity and its importance: results of field experiments with Anthoxanthum odoratum. Oecologia 125:18–25
Shevtsova A, Haukioja E, Ojala A (1997) Growth response of subarctic dwarf shrubs, Empetrum nigrum and Vaccinium vitis-idaea, to manipulated environmental conditions and species removal. Oikos 78:440–458
Smith MD, Knapp AK (2003) Dominant species maintain ecosystem function with non-random species loss. Ecol Lett 6:509–517
SPSS (2006) SPSS for Windows, 15.0th edn. SPSS, Chicago
Steel JB, Wilson JB, Anderson BJ, Lodge RHE, Tangney RS (2004) Are bryophyte communities different from higher-plant communities? Abundance relations. Oikos 104:479–486
Tielborger K, Kadmon R (2000) Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology 81:1544–1553
Totland O, Grytnes JA, Heegaard E (2004) Willow canopies and plant community structure along an alpine environmental gradient. Arctic Antarct Alp Res 36:428–435
Tranquillini W (1980) Winter desiccation as the cause for alpine timberline. In: Benecke U, Davis R (eds) Mountain environments and subalpine tree growth. Forest Research Institute, New Zealand Forest Service, Wellington, pp 263–267
Travis JMJ, Brooker RW, Dytham C (2005) The interplay of positive and negative interactions across an environmental gradient: insights from an individual-based simulation model. Biol Lett 1:5–8
Ugland K, Gray JS (1982) Lognormal distributions and the concept of community equilibrium. Oikos 39:171–178
van Wijk MT et al (2004) Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Global Change Biol 10:105–123
Viereck LA, Little EL (1986) Alaska trees and shrubs. University of Alaska Press, Fairbanks
Weiher E, Keddy PA (1999) Relative abundance and evenness patterns along diversity and biomass gradients. Oikos 87:355–361
Wilby A, Shachak M (2004) Shrubs, granivores and annual plant community stability in an arid ecosystem. Oikos 106:209–216
Wilson JB et al (1996) Are there assembly rules for plant species abundance? An investigation in relation to soil resources and successional trends. J Ecol 84:527–538
Wipf S, Rixen C, Mulder CPH (2006) Advanced snowmelt causes shift towards positive neighbour interactions in a subarctic tundra community. Global Change Biol 12:1496–1506
Zamfir M (2000) Effects of bryophytes and lichens on seedling emergence of alvar plants: evidence from greenhouse experiments. Oikos 88:603–611
Acknowledgments
We are grateful to Sonja Wipf, Tumi Traustason and Jamie Hollingsworth for help with field work. The study was supported by a post-doctoral fellowship [81ZH-068470] from the Swiss National Science Foundation and the Swiss Academy of Sciences. The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mercedes Bustamante.
Rights and permissions
About this article
Cite this article
Rixen, C., Mulder, C.P.H. Species removal and experimental warming in a subarctic tundra plant community. Oecologia 161, 173–186 (2009). https://doi.org/10.1007/s00442-009-1369-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1369-y