Abstract
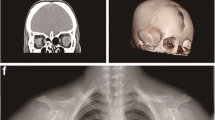
Pediatric intracranial calcification may be caused by inherited or acquired factors. We describe the identification of a novel rearrangement in which a downstream pseudogene translocates into exon 9 of OCLN, resulting in band-like brain calcification and advanced chronic kidney disease in early childhood. SNP genotyping and read-depth variation from whole exome sequencing initially pointed to a mutation in the OCLN gene. The high degree of identity between OCLN and two pseudogenes required a combination of multiplex ligation-dependent probe amplification, PCR, and Sanger sequencing to identify the genomic rearrangement that was the underlying genetic cause of the disease. Mutations in exon 3, or at the 5–6 intron splice site, of OCLN have been reported to cause brain calcification and polymicrogyria with no evidence of extra-cranial phenotypes. Of the OCLN splice variants described, all make use of exon 9, while OCLN variants that use exons 3, 5, and 6 are tissue specific. The genetic rearrangement we identified in exon 9 provides a plausible explanation for the expanded clinical phenotype observed in our individuals. Furthermore, the lack of polymicrogyria associated with the rearrangement of OCLN in our patients extends the range of cranial defects that can be observed due to OCLN mutations.





Similar content being viewed by others
References
Abdel-Salam GM, Zaki MS (2009) Band-like intracranial calcification (BIC), microcephaly and malformation of brain development: a distinctive form of congenital infection like syndromes. Am J Med Genet A 149A(7):1565–1568. doi:10.1002/ajmg.a.32894
Alfares A, Nunez LD, Al-Thihli K, Mitchell J, Melancon S, Anastasio N, Ha KC, Majewski J, Rosenblatt DS, Braverman N (2011) Combined malonic and methylmalonic aciduria: exome sequencing reveals mutations in the ACSF3 gene in patients with a non-classic phenotype. J Med Genet 48(9):602–605. doi:10.1136/jmedgenet-2011-100230
Briggs TA, Wolf NI, D’Arrigo S, Ebinger F, Harting I, Dobyns WB, Livingston JH, Rice GI, Crooks D, Rowland-Hill CA, Squier W, Stoodley N, Pilz DT, Crow YJ (2008) Band-like intracranial calcification with simplified gyration and polymicrogyria: a distinct “pseudo-TORCH” phenotype. Am J Med Genet A 146A(24):3173–3180. doi:10.1002/ajmg.a.32614
Crow YJ, Jackson AP, Roberts E, van Beusekom E, Barth P, Corry P, Ferrie CD, Hamel BC, Jayatunga R, Karbani G, Kalmanchey R, Kelemen A, King M, Kumar R, Livingstone J, Massey R, McWilliam R, Meager A, Rittey C, Stephenson JB, Tolmie JL, Verrips A, Voit T, van Bokhoven H, Brunner HG, Woods CG (2000) Aicardi-Goutieres syndrome displays genetic heterogeneity with one locus (AGS1) on chromosome 3p21. Am J Hum Genet 67(1):213–221. doi:10.1086/302955
Crow YJ, Black DN, Ali M, Bond J, Jackson AP, Lefson M, Michaud J, Roberts E, Stephenson JB, Woods CG, Lebon P (2003) Cree encephalitis is allelic with Aicardi-Goutieres syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J Med Genet 40(3):183–187
Duker AL, Ballif BC, Bawle EV, Person RE, Mahadevan S, Alliman S, Thompson R, Traylor R, Bejjani BA, Shaffer LG, Rosenfeld JA, Lamb AN, Sahoo T (2010) Paternally inherited microdeletion at 15q11.2 confirms a significant role for the SNORD116 C/D box snoRNA cluster in Prader–Willi syndrome. Eur J Hum Genet (EJHG) 18(11):1196–1201. doi:10.1038/ejhg.2010.102
Huqun, Fukuyama S, Morino H, Miyazawa H, Tanaka T, Suzuki T, Kohda M, Kawakami H, Okazaki Y, Seyama K, Hagiwara K (2010) A quantitatively-modeled homozygosity mapping algorithm, qHomozygosityMapping, utilizing whole genome single nucleotide polymorphism genotyping data. BMC Bioinform 11 (Suppl 7):S5. doi:10.1186/1471-2105-11-S7-S5
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25(14):1754–1760. doi:10.1093/bioinformatics/btp324
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20(9):1297–1303. doi:10.1101/gr.107524.110
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628. doi:10.1038/nmeth.1226
O’Driscoll MC, Daly SB, Urquhart JE, Black GC, Pilz DT, Brockmann K, McEntagart M, Abdel-Salam G, Zaki M, Wolf NI, Ladda RL, Sell S, D’Arrigo S, Squier W, Dobyns WB, Livingston JH, Crow YJ (2010) Recessive mutations in the gene encoding the tight junction protein occludin cause band-like calcification with simplified gyration and polymicrogyria. Am J Hum Genet 87(3):354–364. doi:10.1016/j.ajhg.2010.07.012
Olshen AB, Venkatraman ES, Lucito R, Wigler M (2004) Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics 5(4):557–572. doi:10.1093/biostatistics/kxh008
Reardon W, Hockey A, Silberstein P, Kendall B, Farag TI, Swash M, Stevenson R, Baraitser M (1994) Autosomal recessive congenital intrauterine infection-like syndrome of microcephaly, intracranial calcification, and CNS disease. Am J Med Genet 52(1):58–65. doi:10.1002/ajmg.1320520112
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nat Biotechnol 29(1):24–26. doi:10.1038/nbt.1754
Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S (2000) Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11(12):4131–4142
Shi Y, Majewski J (2013) FishingCNV: a graphical software package for detecting rare copy number variations in exome-sequencing data. Bioinformatics. doi:10.1093/bioinformatics/btt151
Thorvaldsdottir H, Robinson JT, Mesirov JP (2012) Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. doi:10.1093/bib/bbs017
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38(16):e164. doi:10.1093/nar/gkq603
Acknowledgments
We are grateful to the family members who generously contributed their time and materials for this research. The authors would like to acknowledge the significant contributions to this work that were made by Dr. Duane Guernsey. Dr. Guernsey was a valued member of our research team who passed during the completion of this project. The following agencies provided funding for this project: Genome Canada, Genome Atlantic, Nova Scotia Health Research Foundation, Nova Scotia Research and Innovation Trust, Dalhousie Faculty of Medicine, Capital District Health Authority, IWK Health Centre Foundation, and Capital Health Research Fund. The authors would like to acknowledge the contribution of the Genome Quebec High Throughput Sequencing Platform. M.A.L is supported by a trainee award from the Beatrice Hunter Cancer Research Institute with funds provided by The Terry Fox Strategic Health Research Training Program in Cancer Research from CIHR. M.E.S is supported by the Centre de Recherche du CHU Ste-Justine.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
M.A. LeBlanc and L.S. Penney contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
LeBlanc, M.A., Penney, L.S., Gaston, D. et al. A novel rearrangement of occludin causes brain calcification and renal dysfunction. Hum Genet 132, 1223–1234 (2013). https://doi.org/10.1007/s00439-013-1327-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-013-1327-y