Abstract
Purpose
Maintaining telomeres by recruiting telomerase-to-chromosome ends is essential for cancer cell survival. Inhibiting telomerase recruitment to telomeres represents a novel strategy for telomere-based lung cancer therapy. However, approaches for interrupting telomerase recruitment for cancer therapy still need to be explored.
Methods
The telomere-binding protein TPP1 is responsible for recruiting telomerase to telomeres and synthesizing telomeres through the association between the oligosaccharide/oligonucleotide-binding (OB)-fold domain of TPP1 and telomerase reverse transcriptase. We overexpressed the TPP1 OB domain (TPP1-OB) by lentivirus infection in lung cancer cells. Telomere length was examined by Southern blot analysis of terminal restriction fragments. The effects of TPP1-OB on cell proliferation, the cell cycle, apoptosis, chemosensitivity, and tumor growth were evaluated in vitro and in vivo.
Result
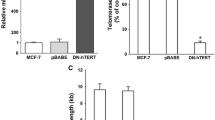
TPP1-OB inhibited the recruitment of telomerase to telomeres and shortened telomere length by acting as a dominant-negative mutant of TPP1. TPP1-OB resulted in reduced cell proliferation, G1 cell cycle arrest, and increased cell apoptosis in lung cancer cells. Cell apoptosis occurred mainly through the caspase-3-dependent signaling pathway. TPP1-OB also suppressed anchorage-independent growth and tumor growth in vivo. Moreover, we demonstrated that TPP1-OB enhances the sensitivity of lung cancer cells to the chemotherapeutic drug paclitaxel.
Conclusion
Our results suggest that inhibiting TPP1-mediated telomerase recruitment by expressing the TPP1-OB domain is a potential novel strategy for telomere-targeted lung cancer therapy.





Similar content being viewed by others
Abbreviations
- OB:
-
Oligosaccharide/oligonucleotide-binding
- TERT:
-
Telomerase reverse transcriptase
- TEL:
-
TPP1 glutamate (E) and leucine (L)-rich
- TEN:
-
Telomerase essential N-terminal
- TIF:
-
Telomere dysfunction-induced foci
- FBS:
-
Fetal bovine serum
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- FISH:
-
Fluorescence in situ hybridization
- PNA:
-
Peptide nucleic acids
References
Abreu E, Aritonovska E, Reichenbach P, Cristofari G, Culp B, Terns RM, Lingner J, Terns MP (2010) TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol Cell Biol 30:2971–2982. https://doi.org/10.1128/Mcb.00240-10
Artandi SE, DePinho RA (2010) Telomeres and telomerase in cancer. Carcinogenesis 31:9–18. https://doi.org/10.1093/carcin/bgp268
Bernadotte A, Mikhelson VM, Spivak IM (2016) Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging-Us 8:3–11. https://doi.org/10.18632/aging.100871
Biroccio A, Gabellini C, Amodei S, Benassi B, Del Bufalo D, Elli R, Antonelli A, D’Incalci M, Zupi G (2003) Telomere dysfunction increases cisplatin and ecteinascidin-743 sensitivity of melanoma cells. Mol Pharmacol 63:632–638
Blackburn EH (2000) Telomere states and cell fates. Nature 408:53–56. https://doi.org/10.1038/35040500
Cech TR (2004) Beginning to understand the end of the chromosome. Cell 116:273–279
Cerone MA, Londono-Vallejo JA, Autexier C (2006) Telomerase inhibition enhances the response to anticancer drug treatment in human breast cancer cells. Mol Cancer Ther 5:1669–1675. https://doi.org/10.1158/1535-7163.MCT-06-0033
Chen C, Gu PL, Wu J, Chen XY, Niu SS, Sun H, Wu LJ, Li N, Peng JH, Shi SH, Fan CY, Huang M, Wong CCL, Gong QG, Kumar-Sinha C, Zhang RG, Pusztai LJ, Rai R, Chang S, Lei M (2017) Structural insights into POT1-TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat Commun. 8:14929
de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110. https://doi.org/10.1101/gad.1346005
Deng YB, Chan SS, Chang S (2008) Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 8:450–458. https://doi.org/10.1038/nrc2393
Dissanayake S, Denny WA, Gamage S, Sarojini V (2017) Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J Control Release 250:62–76. https://doi.org/10.1016/j.jconrel.2017.02.006
Djojosubroto MW, Chin AC, Go N, Schaetzlein S, Manns MP, Gryaznov S, Harley CB, Rudolph KL (2005) Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatoma. Hepatology 42:1127–1136. https://doi.org/10.1002/hep.20822
Grill S, Tesmer VM, Nandakumar J (2018) The N terminus of the OB domain of telomere protein TPP1 is critical for telomerase action. Cell Rep 22(5):1132–1140
Hu C, Rai R, Huang C, Broton C, Long J, Xu Y, Xue J, Lei M, Chang S, Chen Y (2017) Structural and functional analyses of the mammalian TIN2-TPP1-TRF2 telomeric complex. Cell Res 27:1485–1502. https://doi.org/10.1038/cr.2017.144
Lim CJ, Zaug AJ, Kim HJ, Cech TR (2017) Reconstitution of human shelterin complexes reveals unexpected stoichiometry and dual pathways to enhance telomerase processivity. Nat Commun 8:1075. https://doi.org/10.1038/s41467-017-01313-w
Lipinska N, Romaniuk A, Paszel-Jaworska A, Toton E, Kopczynski P, Rubis B (2017) Telomerase and drug resistance in cancer. Cell Mol Life Sci 74:4121–4132. https://doi.org/10.1007/s00018-017-2573-2
Nakashima M, Nandakumar J, Sullivan KD, Espinosa JM, Cech TR (2013) Inhibition of telomerase recruitment and cancer cell death. J Biol Chem 288:33171–33180. https://doi.org/10.1074/jbc.M113.518175
Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR (2012) The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 492:285. https://doi.org/10.1038/nature11648
Palm W, de Lange T (2008) How shelterin protects mammalian telomeres. Annu Rev Genet 42:301–334. https://doi.org/10.1146/annurev.genet.41.110306.130350
Rajavel M, Orban T, Xu MY, Hernandez-Sanchez W, de la Fuente M, Palczewski K, Taylor DJ (2016) Dynamic peptides of human TPP1 fulfill diverse functions in telomere maintenance. Nucleic Acids Res 44:10467–10479. https://doi.org/10.1093/nar/gkw846
Raucher D, Ryu JS (2015) Cell-penetrating peptides: strategies for anticancer treatment. Trends Mol Med 21:560–570. https://doi.org/10.1016/j.molmed.2015.06.00
Riou JF, Guittat L, Mailliet P, Laoui A, Renou E, Petitgenet O, Megnin-Chanet F, Helene C, Mergny JL (2002) Cell senescence and telomere shortening induced by a new series of specific G-quadruplex DNA ligands. Proc Natl Acad Sci USA 99:2672–2677. https://doi.org/10.1073/pnas.052698099
Saretzki G (2003) Telomerase inhibition as cancer therapy. Cancer Lett 194:209–219. https://doi.org/10.1016/S0304-3835(02)00708-5
Schmidt JC, Dalby AB, Cech TR (2014) Identification of human TERT elements necessary for telomerase recruitment to telomeres. Elife. https://doi.org/10.7554/elife.03563
Sexton AN, Regalado SG, Lai CS, Cost GJ, O’Neil CM, Urnov FD, Gregory PD, Jaenisch R, Collins K, Hockemeyer D (2014) Genetic and molecular identification of three human TPP1 functions in telomerase action: recruitment, activation, and homeostasis set point regulation. Genes Dev 28:1885–1899. https://doi.org/10.1101/gad.246819.114
Shay JW (2016) Role of telomeres and telomerase in aging and cancer. Cancer Discov 6:584–593. https://doi.org/10.1158/2159-8290.CD-16-0062
Shay JW, Wright WE (2000) Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol 1:72–76. https://doi.org/10.1038/35036093
Shay JW, Wright WE (2005) Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis 26:867–874. https://doi.org/10.1093/carcin/bgh296
Shay JW, Wright WE (2011) Role of telomeres and telomerase in cancer. Semin Cancer Biol 21:349–353. https://doi.org/10.1016/j.semcancer.2011.10.001
Stewart SA, Weinberg RA (2006) Telomeres: cancer to human aging. Annu Rev Cell Dev Biol 22:531–557. https://doi.org/10.1146/annurev.cellbio.22.010305.104518
Takai KK, Hooper S, Blackwood S, Gandhi R, de Lange T (2010) In vivo stoichiometry of shelterin components. J Biol Chem 285:1457–1467. https://doi.org/10.1074/jbc.M109.038026
Torchilin VP (2014) Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discovery 13:813–827. https://doi.org/10.1038/nrd4333
Wang F, Podell ER, Zaug AJ, Yang YT, Baciu P, Cech TR, Lei M (2007) The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445:506–510. https://doi.org/10.1038/nature0545
Wang Y, Wang X, Flores ER, Yu J, Chang S (2016) Dysfunctional telomeres induce p53-dependent and independent apoptosis to compromise cellular proliferation and inhibit tumor formation. Aging Cell 15:646–660. https://doi.org/10.1111/acel.12476
Ward RJ, Autexier C (2005) Pharmacological telomerase inhibition can sensitize drug-resistant and drug-sensitive cells to chemotherapeutic treatment. Mol Pharmacol 68:779–786. https://doi.org/10.1124/mol.105.011494
Wu D, Gao Y, Qi Y, Chen L, Ma Y, Li Y (2014) Peptide-based cancer therapy: opportunity and challenge. Cancer Lett 351:13–22. https://doi.org/10.1016/j.canlet.2014.05.002
Xin HW, Liu D, Wan M, Safari A, Kim H, Sun W, O’Connor MS, Zhou SY (2007) TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature 445:559–562. https://doi.org/10.1038/nature05469
Zaug AJ, Podell ER, Nandakumar J, Cech TR (2010) Functional interaction between telomere protein TPP1 and telomerase. Genes Dev 24:613–622. https://doi.org/10.1101/gad.1881810
Zhang X, Mar V, Zhou W, Harrington L, Robinson MO (1999) Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev 13:2388–2399
Zhong FL, Batista LFZ, Freund A, Pech MF, Venteicher AS, Artandi SE (2012) TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends. Cell 150:481–494. https://doi.org/10.1016/j.cell.2012.07.012
Funding
This work was supported by the National Natural Science Foundation of China (81472182 to BZ), the Young Innovative Talent Program of Tianjin Medical University Cancer Institute and Hospital (to BZ), and the Tianjin Thousand Talents program (to GG).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, J., Liu, W., Chen, C. et al. TPP1 OB-fold domain protein suppresses cell proliferation and induces cell apoptosis by inhibiting telomerase recruitment to telomeres in human lung cancer cells. J Cancer Res Clin Oncol 145, 1509–1519 (2019). https://doi.org/10.1007/s00432-019-02921-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02921-3