Abstract
Purpose
An immune function assay has been proposed as a new strategy to monitor immunosuppression after organ transplantation. However, there are limited data regarding its role in liver transplant recipients with hepatocellular carcinoma (HCC). In this study, we sought to determine the utility of this functional assay in assessing the risk of infection, rejection, and tumor recurrence in liver transplant recipients.
Methods
Immune function was determined by ImmuKnow assay that measures the amount of adenosine triphosphate (ATP) produced by CD4 (+) T cells to monitor the global immune status in 342 whole blood samples from 105 liver transplant recipients. The association between ATP value and post-transplant tumor recurrence was evaluated in 60 HCC patients. The ATP value in predicting tumor recurrence in other independent cohort of 92 recipients with HCC was analyzed prospectively.
Results
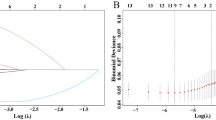
The mean ATP values of liver transplant recipients with infection (145.2 ± 87.0 ng/ml) or acute rejection (418.9 ± 169.5 ng/ml) were different from those with stable state (286.6 ± 143.9 ng/ml, P < 0.05). In recipients with HCC who developed recurrent tumors, the values were significantly lower than those without recurrence (137.8 ± 66.4 vs. 289 ± 133.9 ng/ml, P < 0.01); the optimal threshold value to predict post-transplant tumor recurrence was 175 ng/ml. Comparing with the patients in lower immune group (ATP ≤ 175 ng/ml), patients in the higher immune group (ATP > 175 ng/ml) experienced significantly better disease-free survival (P < 0.01). Multivariate Cox regression analysis showed the ATP value was an independent predictor of HCC recurrence.
Conclusions
The immune function assay has the potential to assess the risk of infection and rejection in liver transplantation and to predict post-transplant tumor recurrence in recipients with HCC.




Similar content being viewed by others
References
Bhorade SM, Janata K, Vigneswaran WT, Alex CG, Garrity ER (2008) Cylex ImmuKnow assay levels are lower in lung transplant recipients with infection. J Heart Lung Transplant 27(9):990–994. doi:10.1016/j.healun.2008.06.005
de Souza AP, Bonorino C (2009) Tumor immunosuppressive environment: effects on tumor-specific and nontumor antigen immune responses. Expert Rev Anticancer Ther 9(9):1317–1332. doi:10.1586/era.09.88
Dirks NL, Huth B, Yates CR, Meibohm B (2004) Pharmacokinetics of immunosuppressants: a perspective on ethnic differences. Int J Clin Pharmacol Ther 42(12):701–718
Gray JW (2003) Evidence emerges for early metastasis and parallel evolution of primary and metastatic tumors. Cancer Cell 4(1):4–6
Gupta S, Mitchell JD, Markham DW, Mammen PP, Patel PC, Kaiser PA, Stastny P, Ring WS, Dimaio JM, Drazner MH (2008) Utility of the Cylex assay in cardiac transplant recipients. J Heart Lung Transplant 27(8):817–822. doi:10.1016/j.healun.2008.05.014
Hashimoto K, Miller C, Hirose K, Diago T, Aucejo F, Quintini C, Eghtesad B, Corey R, Yerian L, Lopez R, Zein N, Fung J (2009) Measurement of CD4+ T-cell function in predicting allograft rejection and recurrent hepatitis C after liver transplantation. Clin Transplant. doi:10.1111/j.1399-0012.2009.01169.x
Husain S, Raza K, Pilewski JM, Zaldonis D, Crespo M, Toyoda Y, Shutt K, Spichty K, Bentlejewski C, Pakstis DL, Carey ME, McCurry KR, Zeevi A (2009) Experience with immune monitoring in lung transplant recipients: correlation of low immune function with infection. Transplantation 87(12):1852–1857. doi:10.1097/TP.0b013e3181a75ad2
Kowalski R, Post D, Schneider MC, Britz J, Thomas J, Deierhoi M, Lobashevsky A, Redfield R, Schweitzer E, Heredia A, Reardon E, Davis C, Bentlejewski C, Fung J, Shapiro R, Zeevi A (2003) Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant 17(2):77–88
Kowalski RJ, Post DR, Mannon RB, Sebastian A, Wright HI, Sigle G, Burdick J, Elmagd KA, Zeevi A, Lopez-Cepero M, Daller JA, Gritsch HA, Reed EF, Jonsson J, Hawkins D, Britz JA (2006) Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation 82(5):663–668. doi:10.1097/01.tp.0000234837.02126.70
Llovet JM, Fuster J, Bruix J (2004) The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl 10(2 Suppl 1):S115–S120. doi:10.1002/lt.20034
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334(11):693–699
Mueller XM (2004) Drug immunosuppression therapy for adult heart transplantation. Part 1: immune response to allograft and mechanism of action of immunosuppressants. Ann Thorac Surg 77(1):354–362
Neff GW, Kemmer N, Kaiser T, Zacharias V, Majoras N, Safdar K (2007) Outcomes in adult and pediatric liver transplantation among various ethnic groups. Transplant Proc 39(10):3204–3206. doi:10.1016/j.transproceed.2007.09.031
Ostrand-Rosenberg S (2008) Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev 18(1):11–18. doi:10.1016/j.gde.2007.12.007
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Perry I, Neuberger J (2005) Immunosuppression: towards a logical approach in liver transplantation. Clin Exp Immunol 139(1):2–10. doi:10.1111/j.1365-2249.2005.02662.x
Rossano JW, Denfield SW, Kim JJ, Price JF, Jefferies JL, Decker JA, Smith EO, Clunie SK, Towbin JA, Dreyer WJ (2009) Assessment of the Cylex ImmuKnow cell function assay in pediatric heart transplant patients. J Heart Lung Transplant 28(1):26–31. doi:10.1016/j.healun.2008.10.001
Rovira P, Mascarell L, Truffa-Bachi P (2000) The impact of immunosuppressive drugs on the analysis of T cell activation. Curr Med Chem 7(7):673–692
Serban G, Whittaker V, Fan J, Liu Z, Manga K, Khan M, Kontogianni K, Padmanabhan A, Cohen D, Suciu-Foca N, Ratner L, Colovai AI (2009) Significance of immune cell function monitoring in renal transplantation after Thymoglobulin induction therapy. Hum Immunol 70(11):882–890. doi:10.1016/j.humimm.2009.07.027
Talmadge JE (2007) Clonal selection of metastasis within the life history of a tumor. Cancer Res 67(24):11471–11475. doi:10.1158/0008-5472.CAN-07-2496
Venkataramanan R, Shaw LM, Sarkozi L, Mullins R, Pirsch J, MacFarlane G, Scheller D, Ersfeld D, Frick M, Fitzsimmons WE, Virji M, Jain A, Brayman KL, Shaked A (2001) Clinical utility of monitoring tacrolimus blood concentrations in liver transplant patients. J Clin Pharmacol 41(5):542–551
Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, Pinna AD (2005) Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl 11(5):497–503. doi:10.1002/lt.20391
Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD (2008) Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg 248(5):857–862. doi:10.1097/SLA.0b013e3181896278
Xue F, Zhang J, Han L, Li Q, Xu N, Zhou T, Xi Z, Wu Y, Xia Q (2010) Immune cell functional assay in monitoring of adult liver transplantation recipients with infection. Transplantation 89(5):620–626. doi:10.1097/TP.0b013e3181c690fa
Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, Simon R, Li Y, Robles AI, Chen Y, Ma ZC, Wu ZQ, Ye SL, Liu YK, Tang ZY, Wang XW (2003) Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med 9(4):416–423. doi:10.1038/nm843
Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, Busuttil RW (2008) Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg 143(2):182–188. doi:10.1001/archsurg.2007.39 (discussion 188)
Acknowledgments
This work is supported by State 863 High Technology R&D Project of China (2007AA02Z479), National Natural Science Foundation of China (30972949), Shanghai Key-Tech Research & Development Program (09411951700), Doctoral Program of Higher Education of China (20090071110022), and National Key Sci-Tech Special Project of China (2008ZX10002-025).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jian-Wen Cheng, Ying-Hong Shi, and Jia Fan contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, JW., Shi, YH., Fan, J. et al. An immune function assay predicts post-transplant recurrence in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol 137, 1445–1453 (2011). https://doi.org/10.1007/s00432-011-1014-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1014-0