Abstract
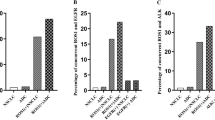
ROS1 has attracted much attention as a possible oncogenic driver and ROS1-rearranged tumors show sensitivity to most ALK inhibitors. We aimed to clarify the prevalence of ROS1 gene rearrangement and investigate the clinical implications of ROS1 gene copy number gain (CNG) in non-small cell lung cancer (NSCLC) patients. We carried out fluorescent in situ hybridization with ROS1 and centromere enumeration 6 probes and immunohistochemistry for ROS1 protein expression. ROS1 rearrangement was detected in 3 of 375 samples (0.8 %); all of whom were female, never-smokers, and harbored an adenocarcinoma component. ROS1 gene CNG was found in 18 cases (4.8 %). ROS1 gene CNG was significantly associated with shorter disease-free survival (DFS, 12 vs. 58 months; p = 0.003) and shorter overall survival (OS, 40 vs. 67 months; p <0.001) than the group without CNG. Multivariate analysis confirmed that ROS1 gene CNG was significantly associated with poorer DFS (hazard ratio [HR]=2.16, 95 % confidence interval [CI] = 1.22–3.81, p = 0.008), and OS ([HR] = 2.53, 95 % [CI] = 1.31–4.89, p = 0.006). ROS1 protein overexpression was observed in 5.0 % (18 out of 357), of which 2 cases harbored ROS1 gene rearrangement. There was no statistically significant correlation between ROS1 gene CNG and protein overexpression. This study demonstrated ROS1 gene rearrangement was detected in 0.8 % of surgically resected NSCLC; and ROS1 gene CNG is an independent poor prognostic factor. This survival analyses may contribute to future studies on the utility of ROS1-targeted therapy for patients.



Similar content being viewed by others
References
Cardarella S, Johnson BE (2013) The impact of genomic changes on treatment of lung cancer. Am J Respir Crit Care Med 188(7):770–775. doi:10.1164/rccm.201305-0843PP
Birchmeier C, Birnbaum D, Waitches G et al (1986) Characterization of an activated human ROS gene. Mol Cell Biol 6(9):3109–3116
Rikova K, Guo A, Zeng Q et al (2007) Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131(6):1190–1203. doi:10.1016/j.cell.2007.11.025
Ou SH, Bartlett CH, Mino-Kenudson M et al (2012) Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 17(11):1351–1375. doi:10.1634/theoncologist. 2012-0311
Yoshida A, Kohno T, Tsuta K et al (2013) ROS1-rearranged lung cancer: a clinicopathologic and molecular study of 15 surgical cases. Am J Surg Pathol 37(4):554–562. doi:10.1097/PAS.0b013e3182758fe6
Robinson DR, Wu YM, Lin SF (2000) The protein tyrosine kinase family of the human genome. Oncogene 19(49):5548–5557. doi:10.1038/sj.onc.1203957
Shaw AT, Hsu PP, Awad MM et al (2013) Tyrosine kinase gene rearrangements in epithelial malignancies. Nat Rev Cancer 13(11):772–787. doi:10.1038/nrc3612
Bergethon K, Shaw AT, Ou SH et al (2012) ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 30(8):863–870. doi:10.1200/JCO.2011.35.6345
Go H, Kim DW, Kim D et al (2013) Clinicopathologic analysis of ROS1-rearranged non-small cell lung cancer and proposal of a diagnostic algorithm. J Thorac Oncol 8(11):1445–1450. doi:10.1097/JTO.0b013e3182a4dd6e
Pan Y, Zhang Y, Li Y et al (2014) ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer 84(2):121–126. doi:10.1016/j.lungcan.2014.02.007
Mescam-Mancini L, Lantuejoul S, Moro-Sibilot D et al (2014) On the relevance of a testing algorithm for the detection of ROS1-rearranged lung adenocarcinomas. Lung Cancer 83(2):168–173. doi:10.1016/j.lungcan.2013.11.019
Kim MH, Shim HS, Kang DR et al (2014) Clinical and prognostic implications of ALK and ROS1 rearrangements in never-smokers with surgically resected lung adenocarcinoma. Lung Cancer 83(3):389–395. doi:10.1016/j.lungcan.2014.01.003
Kim HR, Lim SM, Kim HJ et al (2013) The frequency and impact of ROS1 rearrangement on clinical outcomes in never smokers with lung adenocarcinoma. Ann Oncol 24(9):2364–2370. doi:10.1093/annonc/mdt220
Yoshida A, Tsuta K, Nakamura H et al (2011) Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 35(8):1226–1234. doi:10.1097/PAS.0b013e3182233e06
Edge SB, Byrd DR, Compton CC et al (2010) AJCC cancer staging manual. NY, New York
Travis WD, Brambilla E, Muller-Hermeling HK et al (2004) Pathology and genetics of tumours of the lung, Pleura. IARC Press, Lyon, Thymus and Heart. World Health Organization Classification of Tumours
Travis WD, Brambilla E, Noguchi M et al (2011) International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6(2):244–285. doi:10.1097/JTO.0b013e318206a221
Seo AN, Yang JM, Kim H et al (2014) Clinicopathologic and prognostic significance of c-MYC copy number gain in lung adenocarcinomas. Br J Cancer 110(11):2688–2699. doi:10.1038/bjc.2014.218
Cappuzzo F, Hirsch FR, Rossi E et al (2005) Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small cell lung cancer. J Natl Cancer Inst 97(9):643–655. doi:10.1093/jnci/dji112
Yoshida A, Tsuta K, Wakai S et al (2014) Immunohistochemical detection of ROS1 is useful for identifying ROS1 rearrangements in lung cancers. Mod Pathol 27(5):711–720. doi:10.1038/modpathol.2013.192
Kim H, Yoo SB, Choe JY et al (2011) Detection of ALK gene rearrangement in non-small cell lung cancer: a comparison of fluorescence in situ hybridization and chromogenic in situ hybridization with correlation of ALK protein expression. J Thorac Oncol 6(8):1359–1366. doi:10.1097/JTO.0b013e31821cfc73
Kim H, Jang SJ, Chung DH et al (2013) A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS One 8(10):e76999. doi:10.1371/journal.pone.0076999
Warth A, Muley T, Dienemann H et al (2014) ROS1 expression and translocations in non-small cell lung cancer: clinicopathological analysis of 1478 cases. Histopathology. doi:10.1111/his.12379
Ha SY, Roh MS (2013) The new 2011 international association for the study of lung cancer/American thoracic society/European respiratory society classification of lung adenocarcinoma in resected specimens: clinicopathologic relevance and emerging issues. Korean J Pathol 47(4):316–325. doi:10.4132/KoreanJPathol.2013.47.4.316
Takeuchi K, Soda M, Togashi Y et al (2012) RET, ROS1 and ALK fusions in lung cancer. Nat Med 18(3):378–381. doi:10.1038/nm.2658
Inamura K, Takeuchi K, Togashi Y et al (2009) EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 22(4):508–515. doi:10.1038/modpathol.2009.2
Mackinnon AC Jr, Luevano A, de Araujo LC et al (2014) Cribriform adenocarcinoma of the lung: clinicopathologic, immunohistochemical, and molecular analysis of 15 cases of a distinctive morphologic subtype of lung adenocarcinoma. Mod Pathol. doi:10.1038/modpathol.2013.227
Salido M, Pijuan L, Martinez-Aviles L et al (2011) Increased ALK gene copy number and amplification are frequent in non-small cell lung cancer. J Thorac Oncol 6(1):21–27. doi:10.1097/JTO.0b013e3181fb7cd6
Khadija K, Auger N, Lueza B et al (2012) ALK amplification and crizotinib sensitivity in non-small cell lung cancer cell lines and patients report. J Clin Oncol 30:2012 (suppl; abstr 10556) 30(15)
Camidge DR, Skokan M, Kiatsimkul P et al (2013) Native and rearranged ALK copy number and rearranged cell count in non-small cell lung cancer: implications for ALK inhibitor therapy. Cancer 119(22):3968–3975. doi:10.1002/cncr.28311
Rimkunas VM, Crosby KE, Li D et al (2012) Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res 18(16):4449–4457. doi:10.1158/1078-0432.CCR-11-3351
Lee HJ, Seol HS, Kim JY et al (2013) ROS1 receptor tyrosine kinase, a druggable target, is frequently overexpressed in non-small cell lung carcinomas via genetic and epigenetic mechanisms. Ann Surg Oncol 20(1):200–208. doi:10.1245/s10434-012-2553-6
Acknowledgments
This study was supported by a Grant-in-Aid from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (2013–059757). The authors are indebted to J. Patrick Barron, Professor Emeritus, Tokyo Medical University and Adjunct Professor, Seoul National University Bundang Hospital for his pro bono review of this manuscript.
Conflict of interest
The authors report that they have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, Y., Sun, PL., Kim, H. et al. ROS1 gene rearrangement and copy number gain in non-small cell lung cancer. Virchows Arch 466, 45–52 (2015). https://doi.org/10.1007/s00428-014-1679-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1679-2