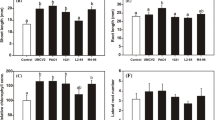
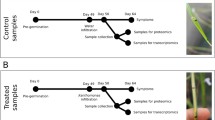
Abstract
Plant root-associated bacteria (rhizobacteria) elicit plant basal immunity referred to as induced systemic resistance (ISR) against multiple pathogens. Among multi-bacterial determinants involving such ISR, the induction of ISR and promotion of growth by bacterial volatile compounds was previously reported. To exploit global de novo expression of plant proteins by bacterial volatiles, proteomic analysis was performed after exposure of Arabidopsis plants to the rhizobacterium Bacillus subtilis GB03. Ethylene biosynthesis enzymes were significantly up-regulated. Analysis by quantitative reverse transcriptase polymerase chain reaction confirmed that ethylene biosynthesis-related genes SAM-2, ACS4, ACS12, and ACO2 as well as ethylene response genes, ERF1, GST2, and CHIB were up-regulated by the exposure to bacterial volatiles. More interestingly, the emission of bacterial volatiles significantly up-regulated both key defense mechanisms mediated by jasmonic acid and salicylic acid signaling pathways. In addition, high accumulation of antioxidant proteins also provided evidence of decreased sensitivity to reactive oxygen species during the elicitation of ISR by bacterial volatiles. The present results suggest that the proteomic analysis of plant defense responses in bacterial volatile-mediated ISR can reveal the mechanisms of plant basal defenses orchestrated by endogenous ethylene production pathways and the generation of reactive oxygen species.




Similar content being viewed by others
Abbreviations
- ET:
-
Ethylene
- ISR:
-
Induced systemic resistance
- JA:
-
Jasmonic acid
- MS:
-
Murashige and Skoog
- PGPR:
-
Plant growth-promoting rhizobacteria
- qRT-PCR:
-
Quantitative RT-PCR
- VOC:
-
Volatile organic compound
- SA:
-
Salicylic acid
References
Agrios GN (2005) Plant pathology. Elsevier Academic Press, pp 635
Anderson NL, Anderson NG (1998) Proteome and proteomics: new technologies, new concepts, and new words. Electrophoresis 19:1853–1861
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Bostock RM (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43:545–580
Camehl I, Sherameti I, Venus Y, Bethke G, Varma A, Lee J, Oelmüller R (2010) Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol 185:1062–1073
Chen Z, Agnew JL, Cohen JD, He P, Shan L, Sheen J, Kunkel BN (2007) Pseudomonas syringae type III effector AvrRpt2 alters Arabidopsis thaliana auxin physiology. Proc Natl Acad Sci USA 104:20131–20136
Chen Z, McConkey BJ, Glick BR (2010) Proteomic studies of plant–bacteria interactions. Soil Biol Biochem. doi:10.1016/j.soilbio.2010.05.033 (in press)
Cho SM, Kang BR, Han SH, Anderson AJ, Park J-Y, Lee Y-H, Cho BH, Yang K-Y, Ryu C-M, Kim YC (2008) 2R, 3R-Butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol Plant Microbe Interact 21:1067–1075
Foyer CH, Lescure JC, Lefebvre C, Morot-Gaudry JF, Vincentz M, Vaucheret H (1994) Adaptations of photosynthetic electron transport, carbon Assimilation, and carbon partitioning in transgenic Nicotiana plumbaginifolia plants to changes in nitrate reductase activity. Plant Physiol 104:171–178
Glick BR (2005) Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett 251:1–7
Halliwell B, Foyer CH (1976) Ascorbic acid, metal ions and the superoxide radical. Biochem J 155:697–700
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Horikawa S, Sasuga J, Shimizu K, Ozasa H, Tsukada K (1990) Molecular cloning and nucleotide sequence of cDNA encoding the rat kidney S-adenosylmethionine synthetase. J Biol Chem 265:13683–13686
Hossain MA, Asada K (1984) Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol 25:85–92
Kalra YP (1998) Handbook of standard methods of plant analysis. CRC Press, Boca Raton, FL
Kandasamy S, Loganathan K, Muthuraj R, Duraisamy S, Seetharaman S, Thiruvengadam R, Ponnusamy B, Ramasamy S (2009) Understanding the molecular basis of plant growth promotional effect of Pseudomonas fluorescens on rice through protein profiling. Proteome Sci 7:47
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can J Bot 65:729–735
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature 286:885–886
Kloepper JW, Rodriguez-Kabana R, Zehnder GW, Murphy J, Sikora E, Fernandez C (1999) Plant root–bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Aust J Plant Pathol 28:27–33
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Lee K, Kye M, Jang JS, Lee OJ, Kim T, Lim D (2004) Proteomic analysis revealed a strong association of a high level of alpha1-antitrypsin in gastric juice with gastric cancer. Proteomics 4:3343–3352
Llorente F, Muskett P, Sánchez-Vallet A, López G, Ramos B, Sánchez-Rodríguez C, Jordá L, Parker J, Molina A (2008) Repression of the auxin response pathway increases Arabidopsis susceptibility to necrotrophic fungi. Mol Plant 1:496–509
Long HH, Sonntag DG, Schmidt DD, Baldwin IT (2010) The structure of the culturable root bacterial endophyte community of Nicotiana attenuata is organized by soil composition and host plant ethylene production and perception. New Phytol 185:554–567
Loper JE, Schroth MN (1986) Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathology 76:386–389
MacDonald EMS, Powell GK, Regier DA, Glass NL, Roberto F, Kosuge T, Morris RO (1986) Secretion of zwatin, ribosylzeatin and ribosyl-1″-methylzeatin by Pseudomonas savastanoi plasmid coded cytokinin biosynthesis. Plant Physiol 82:742–747
McCord JM, Fridovich I (1969) The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol Chem 244:6056–6063
Miché L, Battistoni F, Gemmer S, Belghazi M, Reinhold-Hurek B (2006) Upregulation of jasmonate-inducible defense proteins and differential colonization of roots of Oryza sativa cultivars with the endophyte Azoarcus sp. Mol Plant Microbe Interact 19:502–511
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
Raudales RE, Stone E, McSpadden Gardener BB (2009) Seed treatment with 2,4-diacetylphloroglucinol-producing pseudomonads improves crop health in low pH soils by altering patterns of nutrient uptake. Phytopathology 99:506–511
Robert-Seilaniantz A, Navarro L, Bari R, Jones JD (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10:372–379
Ryu C-M, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100:4927–4932
Ryu C-M, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW (2004a) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Ryu C-M, Murphy JF, Mysore KS, Kloepper JW (2004b) Plant growth-promoting rhizobacteria systemically protect Arabidopsis thaliana against Cucumber mosaic virus by a salicylic acid and NPR1-independent and jasmonic acid-dependent signaling pathway. Plant J 39:381–392
Ryu C-M, Kim J-W, Choi O-H, Park SY, Park SH, Park C-S (2005a) Nature of a root-associated Paenibacillus polymyxa from field-grown winter barley in Korea. J Microbiol Biotechnol 15:984–991
Ryu C-M, Hu C-H, Locy RD, Kloepper JW (2005b) Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil 286:285–292
Ryu C-M, Kim J, Choi O, Kim SH, Park CS (2006) Improvement of biological control capacity of Paenibacillus polymyxa E681 by seed pelleting on sesame. Biol Cont 39:282–289
Segarra G, Casanova E, Bellido D, Odena MA, Oliveira E, Trillas I (2007) Proteome, salicylic acid, and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum strain T34. Proteomics 7:3943–3952
Smith JL, De Moraes CM, Mescher MC (2009) Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest Manag Sci 65:497–503
Timmusk S, Nicander B, Granhall U, Tillberg E (1999) Cytokinin production by Paenibacillus polymyxa. Soil Biol Biochem 31:1847–1852
Truman WM, Bennett MH, Turnbull CG, Grant MR (2010) Arabidopsis auxin mutants are compromised in systemic acquired resistance and exhibit aberrant accumulation of various indolic compounds. Plant Physiol 152:1562–1573
Vallad GE, Goodman RM (2004) Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci 44:1920–1934
Van Loon LC, Geraats BP, Linthorst HJ (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11:184–191
Van Wees SC, de Swart EA, van Pelt JA, van Loon LC, Pieterse CM (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA 97:8711–8716
Varotto C, Maiwald D, Pesaresi P, Jahns P, Salamini F, Leister D (2002) The metal ion transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis in Arabidopsis thaliana. Plant J 31:589–599
Wang W, Tai F, Chen S (2008) Optimizing protein extraction from plant tissues for enhanced proteomics analysis. J Sep Sci 31:2032–2039
Yang JW, Yu SH, Ryu C-M (2009a) Priming of defense-related genes confers root-colonizing bacilli-elicited induced systemic resistance in pepper. Plant Pathol J 25:389–399
Yang J, Kloepper JW, Ryu C-M (2009b) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, Farag MA, Ryu CM, Allen R, Melo IS, Paré PW (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851
Zhang H, Sun Y, Xie X, Kim M-S, Dowd SE, Paré PW (2009a) A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J 58:568–577
Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Paré PW (2009b) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J 56:264–273
Acknowledgments
This work was supported by an EB-NCRC grant (#R15-2003-012-02003-0) and the World Class University Program (#R32-10148) funded by MOEST, the 21C Frontier Microbial Genomics and Application Center Program, KRIBB initiative program, and partly by the Biogreen 21 program (20080401034023/20070401034005) funded by the Rural Development Administration, the Industrial Source Technology Development Program of the Ministry of Knowledge Economy (MKE) of Korea, and the National Academy of Agricultural Science, Rural Development Administration. Y.S.K. was supported by scholarships from the BK21 program funded by MOEST in Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. S. Kwon and C.-M. Ryu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kwon, Y.S., Ryu, CM., Lee, S. et al. Proteome analysis of Arabidopsis seedlings exposed to bacterial volatiles. Planta 232, 1355–1370 (2010). https://doi.org/10.1007/s00425-010-1259-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1259-x