Abstract
Introduction
Subjective cognitive decline (SCD) can represent a preclinical stage of Alzheimer’s disease. Diffusion tensor imaging (DTI) could aid an early diagnosis, yet only few monocentric DTI studies in SCD have been conducted, reporting heterogeneous results. We investigated microstructural changes in SCD in a larger, multicentric cohort.
Methods
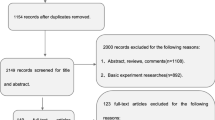
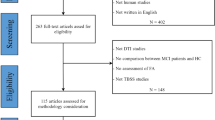
271 participants with SCD, mild cognitive impairment (MCI) or Alzheimer’s dementia (AD) and healthy controls (CON) were included, recruited prospectively at nine centers of the observational DELCODE study. DTI was acquired using identical protocols. Using voxel-based analyses, we investigated fractional anisotropy (FA), mean diffusivity (MD) and mode (MO) in the white matter (WM). Discrimination accuracy was determined by cross-validated elastic-net penalized regression. Center effects were explored using variance analyses.
Results
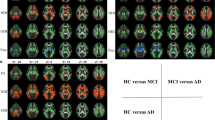
MO and FA were lower in SCD compared to CON in several anterior and posterior WM regions, including the anterior corona radiata, superior and inferior longitudinal fasciculus, cingulum and splenium of the corpus callosum (p < 0.01, uncorrected). MD was higher in the superior and inferior longitudinal fasciculus, cingulum and superior corona radiata (p < 0.01, uncorrected). The cross-validated accuracy for discriminating SCD from CON was 67% (p < 0.01). As expected, the AD and MCI groups had higher MD and lower FA and MO in extensive regions, including the corpus callosum and temporal brain regions. Within these regions, center accounted for 3–15% of the variance.
Conclusions
DTI revealed subtle WM alterations in SCD that were intermediate between those in MCI and CON and may be useful to detect individuals with an increased risk for AD in clinical studies.





Similar content being viewed by others
References
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:270–279
Ashburner J (2007) A fast diffeomorphic image registration algorithm. NeuroImage 38:95–113
Brueggen K, Grothe MJ, Dyrba M, Fellgiebel A, Fischer F, Filippi M, Agosta F, Nestor P, Meisenzahl E, Blautzik J, Frölich L, Hausner L, Bokde ALW, Frisoni G, Pievani M, Klöppel S, Prvulovic D, Barkhof F, Pouwels PJW, Schröder J, Hampel H, Hauenstein K, Teipel S (2017) The European DTI study on dementia—a multicenter DTI and MRI study on Alzheimer’s disease and mild cognitive impairment. NeuroImage 144:305–308
Cherubini A, Péran P, Spoletini I, Di Paola M, Di Iulio F, Hagberg GE, Sancesario G, Gianni W, Bossù P, Caltagirone C, Sabatini U, Spalletta G (2010) Combined volumetry and DTI in subcortical structures of mild cognitive impairment and Alzheimer’s disease patients. J Alzheimers Dis 19:1273–1282
Clerx L, Visser PJ, Verhey F, Aalten P (2012) New MRI markers for Alzheimer’s disease: a meta-analysis of diffusion tensor imaging and a comparison with medial temporal lobe measurements. J Alzheimers Dis 29:405–429
Delbeuck X, van der Linden M, Collette F (2003) Alzheimer’s disease as a disconnection syndrome? Neuropsychol Rev 13:79–92
Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU et al (2011) DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. NeuroImage 55:880–890
Douaud G, Menke RAL, Gass A, Monsch AU, Rao A, Whitcher B, Zamboni G, Matthews PM, Sollberger M, Smith S (2013) Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer’s disease. J Neurosci Off J Soc Neurosci 33:2147–2155
Dyrba M, Barkhof F, Fellgiebel A, Filippi M, Hausner L, Hauenstein K, Kirste T, Teipel SJ (2015) Predicting prodromal Alzheimer’s disease in subjects with mild cognitive impairment using machine learning classification of multimodal multicenter diffusion-tensor and magnetic resonance imaging data. J Neuroimaging Off J Am Soc Neuroimaging 25:738–747
Dyrba M, Ewers M, Wegrzyn M, Kilimann I, Plant C, Oswald A, Meindl T, Pievani M, Bokde ALW, Fellgiebel A, Filippi M, Hampel H, Klöppel S, Hauenstein K, Kirste T, Teipel SJ (2013) Robust automated detection of microstructural white matter degeneration in Alzheimer’s disease using machine learning classification of multicenter DTI data. PLoS ONE 8:e64925
Ennis DB, Kindlmann G (2006) Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med 55:136–146
Fellgiebel A, Wille P, Müller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P (2004) Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord 18:101–108
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. J Psychiatr Res 12:189–198
Frisoni GB, Jack CR Jr, Bocchetta M, Bauer C, Frederiksen KS, Liu Y, Preboske G, Swihart T, Blair M, Cavedo E, Grothe MJ, Lanfredi M, Martinez O, Nishikawa M, Portegies M, Stoub T, Ward C, Apostolova LG, Ganzola R, Wolf D, Barkhof F, Bartzokis G, DeCarli C, Csernansky JG, deToledo-Morrell L, Geerlings MI, Kaye J, Killiany RJ, Lehericy S, Matsuda H, O'Brien J, Silbert LC, Scheltens P, Soininen H, Teipel S, Waldemar G, Fellgiebel A, Barnes J, Firbank M, Gerritsen L, Henneman W, Malykhin N, Pruessner JC, Wang L, Watson C, Wolf H, deLeon M, Pantel J, Ferrari C, Bosco P, Pasqualetti P, Duchesne S, Duvernoy H, Boccardi M, Volumetry E-AWGoTHPfMH, for the Alzheimer’s Disease Neuroimaging I (2015) The EADC-ADNI harmonized protocol for manual hippocampal segmentation on magnetic resonance: evidence of validity. Alzheimers Dement 11:111–125
Gauggel S, Birkner B (1999) Validität und Reliabilität einer deutschen Version der Geriatrischen Depressionsskala (GDS). Zeitschrift für linische Psychologie 28:18–27
Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, Stefano ND, Johansen-Berg H (2010) Age-related changes in grey and white matter structure throughout adulthood. NeuroImage 51:943–951
Grambaite R, Stenset V, Reinvang I, Walhovd KB, Fjell AM, Fladby T (2010) White matter diffusivity predicts memory in patients with subjective and mild cognitive impairment and normal CSF total tau levels. J Int Neuropsychol Soc 16:58–69
Groot Md, Ikram MA, Akoudad S, Krestin GP, Hofman A, van der Lugt A, Niessen WJ, Vernooij MW (2015) Tract-specific white matter degeneration in aging: the Rotterdam Study. Alzheimers Dement J Alzheimers Assoc 11:321–330
Hong YJ, Yoon B, Shim YS, Ahn KJ, Yang DW, Lee J-H (2015) Gray and white matter degenerations in subjective memory impairment: comparisons with normal controls and mild cognitive impairment. J Korean Med Sci 30:1652–1658
Hoy AR, Ly M, Carlsson CM, Okonkwo OC, Zetterberg H, Blennow K, Sager MA, Asthana S, Johnson SC, Alexander AL, Bendlin BB (2017) Microstructural white matter alterations in preclinical Alzheimer’s disease detected using free water elimination diffusion tensor imaging. PLoS ONE 12:e0173982
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, Leon MJD, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de La Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 10:844–852
Jessen F, Spottke A, Boecker H, Brosseron F, Buerger K, Catak C, Fliessbach K, Franke C, Fuentes M, Heneka MT, Janowitz D, Kilimann I, Laske C, Menne F, Nestor P, Peters O, Priller J, Pross V, Ramirez A, Schneider A, Speck O, Spruth EJ, Teipel S, Vukovich R, Westerteicher C, Wiltfang J, Wolfsgruber S, Wagner M, Düzel E (2018) Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimers Res Ther 10:15
Kiuchi K, Kitamura S, Taoka T, Yasuno F, Tanimura M, Matsuoka K, Ikawa D, Toritsuka M, Hashimoto K, Makinodan M, Kosaka J, Morikawa M, Kichikawa K, Kishimoto T (2014) Gray and white matter changes in subjective cognitive impairment, amnestic mild cognitive impairment and Alzheimer’s disease: a voxel-based analysis study. PLoS ONE 9:e104007
Kurth F, Gaser C, Luders E (2015) A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nat Protoc 10:293–304
Li X-Y, Tang Z-C, Sun Y, Tian J, Liu Z-Y, Han Y (2016) White matter degeneration in subjective cognitive decline: a diffusion tensor imaging study. Oncotarget 7:54405–54414
Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, Teipel SJ, Garaci F, Toschi N, Habert M-O, Blennow K, Zetterberg H, O’Bryant SE, Johnson L, Galluzzi S, Bokde ALW, Broich K, Herholz K, Bakardjian H, Dubois B, Jessen F, Carrillo MC, Aisen PS, Hampel H (2015) Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline. J Alzheimers Dis 48(Suppl 1):S171–S191
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40:570–582
Müller MJ, Greverus D, Weibrich C, Dellani PR, Scheurich A, Stoeter P, Fellgiebel A (2007) Diagnostic utility of hippocampal size and mean diffusivity in amnestic MCI. Neurobiol Aging 28:398–403
Ryu SY, Lim EY, Na S, Shim YS, Cho JH, Yoon B, Hong YJ, Yang DW (2017) Hippocampal and entorhinal structures in subjective memory impairment: a combined MRI volumetric and DTI study. Int Psychogeriatr 29:1–8
Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM (2005) Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci 1064:37–49
Selnes P, Aarsland D, Bjørnerud A, Gjerstad L, Wallin A, Hessen E, Reinvang I, Grambaite R, Auning E, Kjærvik VK, Due-Tønnessen P, Stenset V, Fladby T (2013) Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. J Alzheimers Dis 33:723–736
Selnes P, Fjell AM, Gjerstad L, Bjornerud A, Wallin A, Due-Tonnessen P, Grambaite R, Stenset V, Fladby T (2012) White matter imaging changes in subjective and mild cognitive impairment. Alzheimers Dement J Alzheimers Assoc 8:S112–S121
Soares JM, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci 7:31
Stenset V, Bjørnerud A, Fjell AM, Walhovd KB, Hofoss D, Due-Tønnessen P, Gjerstad L, Fladby T (2011) Cingulum fiber diffusivity and CSF T-tau in patients with subjective and mild cognitive impairment. Neurobiol Aging 32:581–589
Teipel SJ, Grothe MJ, Filippi M, Fellgiebel A, Dyrba M, Frisoni GB, Meindl T, Bokde ALW, Hampel H, Klöppel S, Hauenstein K, The EDSD Study Group (2014) Fractional anisotropy changes in Alzheimer’s disease depend on the underlying fiber tract architecture: a multiparametric DTI study using joint independent component analysis. J Alzheimers Dis 41:69–83
Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein K-H, Filippi M, Ernemann U, Reiser MF, Hampel H (2010) Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis 22:507–522
Teipel SJ, Reuter S, Stieltjes B, Acosta-Cabronero J, Ernemann U, Fellgiebel A, Filippi M, Frisoni G, Hentschel F, Jessen F, Klöppel S, Meindl T, Pouwels PJW, Hauenstein K-H, Hampel H (2011) Multicenter stability of diffusion tensor imaging measures: a European clinical and physical phantom study. Psychiatry Res 194:363–371
Teipel SJ, Wegrzyn M, Meindl T, Frisoni G, Bokde ALW, Fellgiebel A, Filippi M, Hampel H, Kloppel S, Hauenstein K, Ewers M (2012) Anatomical MRI and DTI in the diagnosis of Alzheimer’s disease: a European multicenter study. J Alzheimers Dis 31(Suppl 3):S33–S47
Wang Y, West JD, Flashman LA, Wishart HA, Santulli RB, Rabin LA, Pare N, Arfanakis K, Saykin AJ (2012) Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim Biophys Acta 1822:423–430
Winston GP (2012) The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2:254–265
Zhou H, Hastie T (2005) Regularization and variable selection via the elastic net. J R Stat Soc B 67(part 2):301–320
Zou H, Hastie T (2005) Regularization and variable selection via the elastic net. J R Stat Soc Ser B (Stat Methodol) 67:301–320
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study has been approved by the local institutional review boards and ethics committees of the participating centers. It has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brueggen, K., Dyrba, M., Cardenas-Blanco, A. et al. Structural integrity in subjective cognitive decline, mild cognitive impairment and Alzheimer’s disease based on multicenter diffusion tensor imaging. J Neurol 266, 2465–2474 (2019). https://doi.org/10.1007/s00415-019-09429-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09429-3