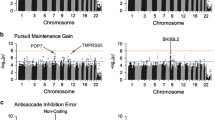
Abstract
Similar smooth pursuit eye tracking dysfunctions are present across psychotic disorders. They include pursuit initiation and maintenance deficits that implicate different functional brain systems. This candidate gene study examined psychosis-related genotypes regulating dopamine and glutamate neurotransmission in relation to these pursuit deficits. One hundred and thirty-eight untreated first-episode patients with a psychotic disorder were genotyped for four markers in DRD2 and four markers in GRM3. The magnitude of eye movement abnormality in patients was defined in relation to performance of matched healthy controls (N = 130). Eighty three patients were followed after 6 weeks of antipsychotic treatment. At baseline, patients with a −141C deletion in DRD2 rs1799732 had slower initiation eye velocity and longer pursuit latency than CC insertion carriers. Further, GRM3 rs274622_CC carriers had poorer pursuit maintenance than T-carriers. Antipsychotic treatment resulted in prolonged pursuit latency in DRD2 rs1799732_CC insertion carriers and a decline in pursuit maintenance in GRM3 rs6465084_GG carriers. The present study demonstrates for the first time that neurophysiological measures of motor and neurocognitive deficits in patients with psychotic disorders have different associations with genes regulating dopamine and glutamate systems, respectively. Alterations in striatal D2 receptor activity through the −141C Ins/Del polymorphism could contribute to pursuit initiation deficits in psychotic disorders. Alterations in GRM3 coding for the mGluR3 protein may impair pursuit maintenance by compromising higher perceptual and cognitive processes that depend on optimal glutamate signaling in corticocortical circuits. DRD2 and GRM3 genotypes also selectively modulated the severity of adverse motor and neurocognitive changes resulting from antipsychotic treatment.




Similar content being viewed by others
References
Gottesman II, Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160(4):636–645
Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M (2008) Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev 7(1):68–83
Holzman PS (1992) Behavioral markers of schizophrenia useful for genetic studies. J Psychiatr Res 26(4):427–445
Thaker GK (2008) Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull 34(4):760–773
Lencer R, Malchow CP, Krecker K, Nolte A, Pinnow M, von Siefart SZ, Schwinger E, Arolt V (1999) Smooth pursuit performance in families with multiple occurrence of schizophrenia and nonpsychotic families. Biol Psychiatry 45(6):694–703
Levy DL, Sereno AB, Gooding DC, O’Driscoll GA (2010) Eye tracking dysfunction in schizophrenia: characterization and pathophysiology. Curr Top Behav Neurosci 4:311–347
Sweeney JA, Clementz BA, Haas GL, Escobar MD, Drake K, Frances AJ (1994) Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity, and the role of attention. J Abnorm Psychol 103(2):222–230
Calkins ME, Iacono WG, Ones DS (2008) Eye movement dysfunction in first-degree relatives of patients with schizophrenia: a meta-analytic evaluation of candidate endophenotypes. Brain Cogn 68(3):436–461
Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O’Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O’Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T (2003) Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet 73(1):34–48. doi:10.1086/376549
Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM (2009) Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 373(9659):234–239
Williams HJ, Craddock N, Russo G, Hamshere ML, Moskvina V, Dwyer S, Smith RL, Green E, Grozeva D, Holmans P, Owen MJ, O’Donovan MC (2011) Most genome-wide significant susceptibility loci for schizophrenia and bipolar disorder reported to date cross-traditional diagnostic boundaries. Hum Mol Genet 20(2):387–391. doi:10.1093/hmg/ddq471
McIntosh AM, Moorhead TW, McKirdy J, Hall J, Sussmann JE, Stanfield AC, Harris JM, Johnstone EC, Lawrie SM (2009) Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiatr Scand 119(3):192–198
McIntosh AM, Whalley HC, McKirdy J, Hall J, Sussmann JE, Shankar P, Johnstone EC, Lawrie SM (2008) Prefrontal function and activation in bipolar disorder and schizophrenia. Am J Psychiatry 165(3):378–384
Lencer R, Reilly JL, Harris MS, Sprenger A, Keshavan MS, Sweeney JA (2010) Sensorimotor transformation deficits for smooth pursuit in first-episode affective psychoses and schizophrenia. Biol Psychiatry 67(3):217–223
Lencer R, Trillenberg P, Trillenberg-Krecker K, Junghanns K, Kordon A, Broocks A, Hohagen F, Heide W, Arolt V (2004) Smooth pursuit deficits in schizophrenia, affective disorder and obsessive-compulsive disorder. Psychol Med 34(3):451–460
Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME (1999) Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry 46(5):671–680
Blackwood DH, Sharp CW, Walker MT, Doody GA, Glabus MF, Muir WJ (1996) Implications of comorbidity for genetic studies of bipolar disorder: P300 and eye tracking as biological markers for illness. Br J Psychiatry Suppl 30:85–92
Kathmann N, Hochrein A, Uwer R, Bondy B (2003) Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. Am J Psychiatry 160(4):696–702
Lencer R, Sprenger A, Harris MS, Reilly JL, Keshavan MS, Sweeney JA (2008) Effects of second-generation antipsychotic medication on smooth pursuit performance in antipsychotic-naive schizophrenia. Arch Gen Psychiatry 65(10):1146–1154
Hutton SB, Crawford TJ, Gibbins H, Cuthbert I, Barnes TR, Kennard C, Joyce EM (2001) Short and long term effects of antipsychotic medication on smooth pursuit eye tracking in schizophrenia. Psychopharmacology 157(3):284–291
Sweeney JA, Haas GL, Li S, Weiden PJ (1994) Selective effects of antipsychotic medications on eye-tracking performance in schizophrenia. Psychiatry Res 54(2):185–198
Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, Carl JR (1998) Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biol Psychiatry 44(8):698–708
Lencer R, Trillenberg P (2008) Neurophysiology and neuroanatomy of smooth pursuit in humans. Brain Cogn 68(3):219–228
Helmchen C, Pohlmann J, Trillenberg P, Lencer R, Graf J, Sprenger A (2012) Role of anticipation and prediction in smooth pursuit eye movement control in Parkinson’s disease. Mov Disord 27(8):1012–1018. doi:10.1002/mds.25042
Lencer R, Nagel M, Sprenger A, Zapf S, Erdmann C, Heide W, Binkofski F (2004) Cortical mechanisms of smooth pursuit eye movements with target blanking. An fMRI study. Eur J Neurosci 19(5):1430–1436
Burke MR, Barnes GR (2008) Brain and behavior: a task-dependent eye movement study. Cereb Cortex 18(1):126–135
Marenco S, Steele SU, Egan MF, Goldberg TE, Straub RE, Sharrief AZ, Weinberger DR (2006) Effect of metabotropic glutamate receptor 3 genotype on N-acetylaspartate measures in the dorsolateral prefrontal cortex. Am J Psychiatry 163(4):740–742. doi:10.1176/appi.ajp.163.4.740
Haraldsson HM, Ettinger U, Magnusdottir BB, Sigmundsson T, Sigurdsson E, Ingason A, Petursson H (2009) COMT val(158)met genotype and smooth pursuit eye movements in schizophrenia. Psychiatry Res 169(2):173–175. doi:10.1016/j.psychres.2008.10.003
Vorstman JA, Turetsky BI, Sijmens-Morcus ME, de Sain MG, Dorland B, Sprong M, Rappaport EF, Beemer FA, Emanuel BS, Kahn RS, van Engeland H, Kemner C (2009) Proline affects brain function in 22q11DS children with the low activity COMT 158 allele. Neuropsychopharmacology 34(3):739–746. doi:10.1038/npp.2008.132
Park BL, Shin HD, Cheong HS, Park CS, Sohn JW, Kim BJ, Seo HK, Kim JW, Kim KH, Shin TM, Choi IG, Kim SG, Woo SI (2009) Association analysis of COMT polymorphisms with schizophrenia and smooth pursuit eye movement abnormality. J Hum Genet 54(12):709–712. doi:10.1038/jhg.2009.102
Rybakowski JK, Borkowska A, Czerski PM, Hauser J (2001) Dopamine D3 receptor (DRD3) gene polymorphism is associated with the intensity of eye movement disturbances in schizophrenic patients and healthy subjects. Mol Psychiatry 6(6):718–724
Rybakowski JK, Borkowska A, Czerski PM, Hauser J (2002) Eye movement disturbances in schizophrenia and a polymorphism of catechol-O-methyltransferase gene. Psychiatry Res 113(1–2):49–57
Thaker GK, Wonodi I, Avila MT, Hong LE, Stine OC (2004) Catechol O-methyltransferase polymorphism and eye tracking in schizophrenia: a preliminary report. Am J Psychiatry 161(12):2320–2322
Wonodi I, Hong LE, Stine OC, Mitchell BD, Elliott A, Roberts RC, Conley RR, McMahon RP, Thaker GK (2009) Dopamine transporter polymorphism modulates oculomotor function and DAT1 mRNA expression in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 150B(2):282–289
Haraldsson HM, Ettinger U, Magnusdottir BB, Ingason A, Hutton SB, Sigmundsson T, Sigurdsson E, Petursson H (2010) Neuregulin-1 genotypes and eye movements in schizophrenia. Eur Arch Psychiatry Clin Neurosci 260(1):77–85. doi:10.1007/s00406-009-0032-2
Pasaje CF, Bae JS, Park BL, Cheong HS, Kim JH, Park TJ, Lee JS, Kim Y, Park CS, Kim BJ, Cha B, Kim JW, Choi WH, Shin TM, Choi IG, Hwang J, Shin HD, Woo SI (2011) Neuregulin 3 does not confer risk for schizophrenia and smooth pursuit eye movement abnormality in a Korean population. Genes, brain, behav 10(8):828–833. doi:10.1111/j.1601-183X.2011.00722.x
Cheong HS, Park BL, Kim EM, Park CS, Sohn JW, Kim BJ, Kim JW, Kim KH, Shin TM, Choi IG, Han SW, Hwang J, Koh I, Shin HD, Woo SI (2011) Association of RANBP1 haplotype with smooth pursuit eye movement abnormality. Am J Med Genet B Neuropsychiatr Genet 156B(1):67–71. doi:10.1002/ajmg.b.31139
Shin HD, Park BL, Bae JS, Park TJ, Chun JY, Park CS, Sohn JW, Kim BJ, Kang YH, Kim JW, Kim KH, Shin TM, Woo SI (2010) Association of ZDHHC8 polymorphisms with smooth pursuit eye movement abnormality. Am J Med Genet B Neuropsychiatr Genet 153B(6):1167–1172. doi:10.1002/ajmg.b.31083
Arolt V, Lencer R, Purmann S, Schurmann M, Muller-Myhsok B, Krecker K, Schwinger E (1999) Testing for linkage of eye tracking dysfunction and schizophrenia to markers on chromosomes 6, 8, 9, 20, and 22 in families multiply affected with schizophrenia. Am J Med Genet 88(6):603–606
Pasaje CF, Bae JS, Park BL, Park CS, Kim BJ, Lee CS, Kim JW, Choi WH, Shin TM, Koh IS, Choi IG, Woo SL, Shin HD (2011) Lack of association of the RTN4R genetic variations with risk of schizophrenia and SPEM abnormality in a Korean population. Psychiatry Res 189(2):312–314. doi:10.1016/j.psychres.2011.02.006
Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R (2011) Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry 68(7):665–674. doi:10.1001/archgenpsychiatry.2011.71
Jonsson EG, Nothen MM, Neidt H, Forslund K, Rylander G, Mattila-Evenden M, Asberg M, Propping P, Sedvall GC (1999) Association between a promoter polymorphism in the dopamine D2 receptor gene and schizophrenia. Schizophr Res 40(1):31–36
Zhang JP, Lencz T, Malhotra AK (2010) D2 receptor genetic variation and clinical response to antipsychotic drug treatment: a meta-analysis. Am J Psychiatry 167(7):763–772. doi:10.1176/appi.ajp.2009.09040598
Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, Akil M, Crook J, Vakkalanka RK, Balkissoon R, Gibbs RA, Kleinman JE, Weinberger DR (2004) Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci USA 101(34):12604–12609. doi:10.1073/pnas.0405077101
Cherlyn SY, Woon PS, Liu JJ, Ong WY, Tsai GC, Sim K (2010) Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: a decade of advance. Neurosci Biobehav Rev 34(6):958–977. doi:10.1016/j.neubiorev.2010.01.002
Ghose S, Gleason KA, Potts BW, Lewis-Amezcua K, Tamminga CA (2009) Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: a mechanism for antipsychotic drug action? Am J Psychiatry 166(7):812–820. doi:10.1176/appi.ajp.2009.08091445
Baune BT, Suslow T, Beste C, Birosova E, Domschke K, Sehlmeyer C, Konrad C (2010) Association between genetic variants of the metabotropic glutamate receptor 3 (GRM3) and cognitive set shifting in healthy individuals. Genes, brain, behav 9(5):459–466. doi:10.1111/j.1601-183X.2010.00573.x
Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M (2013) Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science (New York, NY) 339(6116):197–200. doi:10.1126/science.1226740
Corripio I, Ferreira A, Portella MJ, Perez V, Escarti MJ, Del Valle Camacho M, Sauras RB, Alonso A, Grasa EM, Carrio I, Catafau AM, Alvarez E (2012) The role of striatal dopamine D2 receptors in the occurrence of extrapyramidal side effects: iodine-123-iodobenzamide single photon emission computed tomography study. Psychiatry Res 201(1):73–77. doi:10.1016/j.pscychresns.2011.02.004
Bishop JR, Ellingrod VL, Moline J, Miller D (2005) Association between the polymorphic GRM3 gene and negative symptom improvement during olanzapine treatment. Schizophr Res 77(2–3):253–260. doi:10.1016/j.schres.2005.04.001
Fijal BA, Kinon BJ, Kapur S, Stauffer VL, Conley RR, Jamal HH, Kane JM, Witte MM, Houston JP (2009) Candidate-gene association analysis of response to risperidone in African-American and white patients with schizophrenia. Pharmacogenomics J 9(5):311–318. doi:10.1038/tpj.2009.24
First MB, Spitzer RL, Gibbon M, Williams JBW (1995) Structured clinical interview for DSM-IV axis I disorders, patient edition (SCID-P). New York State Psychiatric Institute, New York
Rhoades HM, Overall JE (1988) The semistructured BPRS interview and rating guide. Psychopharmacol Bull 24(1):101–104
The Psychological Corporation TP (1999) Wechsler abbreviated scale of intelligence (WASI) manual. The Psychological Corporation, San Antonio, TX
Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC (2010) Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 67(3):255–262. doi:10.1016/j.biopsych.2009.08.040
Bishop JR, del Miller D, Ellingrod VL, Holman T (2011) Association between type-three metabotropic glutamate receptor gene (GRM3) variants and symptom presentation in treatment refractory schizophrenia. Hum Psychopharmacol 26(1):28–34. doi:10.1002/hup.1163
Houston J, Dharia S, Bishop JR, Ellingrod VL, Fijal B, Jacobson JG, Hoffmann VP (2011) Association of DRD2 and ANKK1 polymorphisms with prolactin increase in olanzapine-treated women. Psychiatry Res 187(1–2):74–79. doi:10.1016/j.psychres.2010.10.020
Lencz T, Robinson DG, Napolitano B, Sevy S, Kane JM, Goldman D, Malhotra AK (2010) DRD2 promoter region variation predicts antipsychotic-induced weight gain in first episode schizophrenia. Pharmacogenet Genomics 20(9):569–572. doi:10.1097/FPC.0b013e32833ca24b
Tian C, Hinds DA, Shigeta R, Kittles R, Ballinger DG, Seldin MF (2006) A genomewide single-nucleotide-polymorphism panel with high ancestry information for African American admixture mapping. Am J Hum Genet 79(4):640–649
Giri VN, Egleston B, Ruth K, Uzzo RG, Chen DY, Buyyounouski M, Raysor S, Hooker S, Torres JB, Ramike T, Mastalski K, Kim TY, Kittles R (2009) Race, genetic West African ancestry, and prostate cancer prediction by prostate-specific antigen in prospectively screened high-risk men. Cancer Prev Res (Phila Pa) 2(3):244–250
Hooker S, Hernandez W, Chen H, Robbins C, Torres JB, Ahaghotu C, Carpten J, Kittles RA (2010) Replication of prostate cancer risk loci on 8q24, 11q13, 17q12, 19q33, and Xp11 in African Americans. Prostate 70(3):270–275. doi:10.1002/pros.21061
Kupfer SS, Anderson JR, Hooker S, Skol A, Kittles RA, Keku TO, Sandler RS, Ellis NA (2010) Genetic heterogeneity in colorectal cancer associations in Americans of African vs European Descent. Gastroenterology. doi:10.1053/j.gastro.2010.07.038
Kupfer SS, Torres JB, Hooker S, Anderson JR, Skol AD, Ellis NA, Kittles RA (2009) Novel single nucleotide polymorphism associations with colorectal cancer on chromosome 8q24 in African and European Americans. Carcinogenesis 30(8):1353–1357. doi:10.1093/carcin/bgp123
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164(4):1567–1587
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575. doi:10.1086/519795
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21(2):263–265. doi:10.1093/bioinformatics/bth457
Arinami T, Gao M, Hamaguchi H, Toru M (1997) A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet 6(4):577–582
Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC (1999) Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry 4(3):290–296
Cordeiro Q, Siqueira-Roberto J, Zung S, Vallada H (2009) Association between the DRD2−141C insertion/deletion polymorphism and schizophrenia. Arq Neuropsiquiatr 67(2A):191–194
Li T, Arranz M, Aitchison KJ, Bryant C, Liu X, Kerwin RW, Murray R, Sham P, Collier DA (1998) Case-control, haplotype relative risk and transmission disequilibrium analysis of a dopamine D2 receptor functional promoter polymorphism in schizophrenia. Schizophr Res 32(2):87–92
Stober G, Jatzke S, Heils A, Jungkunz G, Knapp M, Mossner R, Riederer P, Lesch KP (1998) Insertion/deletion variant (−141C Ins/Del) in the 5′ regulatory region of the dopamine D2 receptor gene: lack of association with schizophrenia and bipolar affective disorder. Short communication. J neural transm 105(1):101–109
Lencer R, Keedy SK, Reilly JL, McDonough BE, Harris MS, Sprenger A, Sweeney JA (2011) Altered transfer of visual motion information to parietal association cortex in untreated first-episode psychosis: implications for pursuit eye tracking. Psychiatry Res 194(1):30–38
Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. doi:10.1146/annurev.pharmtox.37.1.205
Bishop JR, Wang K, Moline J, Ellingrod VL (2007) Association analysis of the metabotropic glutamate receptor type 3 gene (GRM3) with schizophrenia. Psychiatr Genet 17(6):358. doi:10.1097/YPG.0b013e3281ac231e
Dalvie S, Horn N, Nossek C, van der Merwe L, Stein DJ, Ramesar R (2010) Psychosis and relapse in bipolar disorder are related to GRM3, DAOA, and GRIN2B genotype. Afr J Psychiatry 13(4):297–301
Kawakubo Y, Suga M, Tochigi M, Yumoto M, Itoh K, Sasaki T, Kano Y, Kasai K (2011) Effects of metabotropic glutamate receptor 3 genotype on phonetic mismatch negativity. PLoS ONE 6(10):e24929. doi:10.1371/journal.pone.0024929
Corti C, Xuereb JH, Corsi M, Ferraguti F (2001) Identification and characterization of the promoter region of the GRM3 gene. Biochem Biophys Res Commun 286(2):381–387. doi:10.1006/bbrc 2001.5391
Fujii Y, Shibata H, Kikuta R, Makino C, Tani A, Hirata N, Shibata A, Ninomiya H, Tashiro N, Fukumaki Y (2003) Positive associations of polymorphisms in the metabotropic glutamate receptor type 3 gene (GRM3) with schizophrenia. Psychiatr Genet 13(2):71–76. doi:10.1097/01.ypg.0000056682.82896.b0
Mossner R, Schuhmacher A, Schulze-Rauschenbach S, Kuhn KU, Rujescu D, Rietschel M, Zobel A, Franke P, Wolwer W, Gaebel W, Hafner H, Wagner M, Maier W (2008) Further evidence for a functional role of the glutamate receptor gene GRM3 in schizophrenia. Eur Neuropsychopharmacol 18(10):768–772. doi:10.1016/j.euroneuro.2008.05.007
Schwab SG, Plummer C, Albus M, Borrmann-Hassenbach M, Lerer B, Trixler M, Maier W, Wildenauer DB (2008) DNA sequence variants in the metabotropic glutamate receptor 3 and risk to schizophrenia: an association study. Psychiatr Genet 18(1):25–30. doi:10.1097/YPG.0b013e3282ef48d9
Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, Weinberger DR (2003) Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry 160(4):709–719
Nagel M, Sprenger A, Nitschke M, Zapf S, Heide W, Binkofski F, Lencer R (2007) Different extraretinal neuronal mechanisms of smooth pursuit eye movements in schizophrenia: an fMRI study. Neuroimage 34(1):300–309
Acknowledgments
We thank Drs. Ovidio DeLeon, Gretchen Haas, Robert Marvin, Debra Montrose, Cherise Rosen, Hugo Solari, Peter Weiden and the clinical core staff of the Center for the Neuroscience of Mental Disorders (MH45156, David Lewis MD, Director) for their contributions to diagnostic and psychopathological assessments. We note with appreciation the general statistical genetics input to our studies by Dr. Judith Badner. This study was supported by National Institute of Health (NIH) grants MH083888, MH062134, MH083126, MH45156, MH63480, RR024153, CTSA Grant UL1TR000050 and NIH/NCRR/GCRC Grant RR00056, Janssen Pharmaceuticals and the Alexander von Humboldt Foundation. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of NIH. Main results of this study have been presented as a poster at the World Congress of Psychiatric Genetics 2012 in Hamburg, Germany.
Conflict of interest
Dr Sweeney is a consultant to Roche, Pfizer, Takeda, Bristol-Myers Squibb and Eli Lilly. Dr. Bishop has received research support from Ortho-McNeil Janssen. Drs Lencer, Harris, Reilly, Patel, Kittles, Prasad, Nimgaonkar and Keshavan report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lencer, R., Bishop, J.R., Harris, M.S.H. et al. Association of variants in DRD2 and GRM3 with motor and cognitive function in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci 264, 345–355 (2014). https://doi.org/10.1007/s00406-013-0464-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-013-0464-6