Abstract
Purpose
Polycystic ovary syndrome (PCOS) is one of the most common endocrinopathies that affects women in reproductive age. MicroRNAs (miRNAs) play crucial roles in normal function of female reproductive system and folliculogenesis. Deregulated expression of miRNAs in PCOS condition may be significantly implicated in the pathogenesis of PCOS. We determined relative expression of miR-15a, miR-145, and miR-182 in granulosa-lutein cells (GLCs), follicular fluid (FF), and serum of PCOS patients.
Methods
Human subjects were divided into PCOS (n = 20) and control (n = 21) groups. GLCs, FF, and serum were isolated and stored. RNA isolation was performed and cDNA was reversely transcribed using specific stem-loop RT primers. Relative expression of miRNAs was calculated after normalization against U6 expression. Correlation of miRNAs’ expression level with basic clinical features and predictive value of miRNAs in FF and serum were appraised.
Results
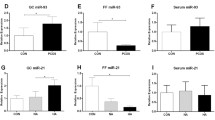
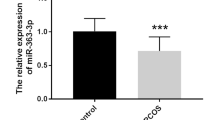
Relative expression of miR-145 and miR-182 in GLCs was significantly decreased in PCOS, but miR-182 in FF of PCOS patients revealed up-regulated levels. Significant correlations between level of miRNAs in FF and serum and hormonal profile of subjects were observed. MiR-182 in FF showed a significant predictive value with AUC of 0.73, 76.4% sensitivity, and 70.5% specificity which was improved after combination of miR-182 and miR-145.
Conclusions
A significant dysregulation of miR-145 and miR-182 in GLCs of PCOS may indicate their involvement in pathogenesis of PCOS. Differential up-regulation of miR-182 in FF of PCOS patients with its promising predictive values for discrimination of PCOS reinforced the importance of studying miRNAs’ profile in FF.




Similar content being viewed by others
References
Franks S (1995) Polycystic ovary syndrome. N Engl J Med 333(13):853–861
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R (2011) Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 7(4):219–231
Hughesdon P (1982) Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called” hyperthecosis”. Obstet Gynecol Surv 37(2):59–77
Bennett J, Wu Y-G, Gossen J, Zhou P, Stocco C (2012) Loss of GATA-6 and GATA-4 in granulosa cells blocks folliculogenesis, ovulation, and follicle stimulating hormone receptor expression leading to female infertility. Endocrinology 153(5):2474–2485
Pelusi C, Ikeda Y, Zubair M, Parker KL (2008) Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells1. Biol Reprod 79(6):1074–1083
Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S (1998) Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation 1. J Clin Endocrinol Metab 83(11):3984–3991
Coskun S, Otu HH, Awartani KA, Al-Alwan LA, Al-Hassan S, Al-Mayman H, Kaya N, Inan MS (2013) Gene expression profiling of granulosa cells from PCOS patients following varying doses of human chorionic gonadotropin. J Assist Reprod Genet 30(3):341–352
Shalev E, Goldman S, Ben-Shlomo I (2001) The balance between MMP-9 and MMP-2 and their tissue inhibitor (TIMP)-1 in luteinized granulosa cells: comparison between women with PCOS and normal ovulatory women. Mol Hum Reprod 7(4):325–331
Kawamata T, Tomari Y (2010) Making risc. Trends Biochem Sci 35(7):368–376
Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N (2008) Widespread changes in protein synthesis induced by microRNAs. Nature 455(7209):58–63
Li Y, Fang Y, Liu Y, Yang X (2015) MicroRNAs in ovarian function and disorders. J Ovar Res 8(1):51
Sørensen AE, Wissing ML, Salö S, Englund ALM, Dalgaard LT (2014) MicroRNAs related to polycystic ovary syndrome (PCOS). Genes 5(3):684–708
Teague EMCO, Hull ML (2010) The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update 16(2):142–165
Karakaya C, Guzeloglu-Kayisli O, Uyar A, Kallen AN, Babayev E, Bozkurt N, Unsal E, Karabacak O, Seli E (2015) Poor ovarian response in women undergoing in vitro fertilization is associated with altered microRNA expression in cumulus cells. Fertil Steril 103(6):1469–1476 (e1463)
Sørensen AE, Wissing ML, Englund ALM, Dalgaard LT (2016) MicroRNA species in follicular fluid associating with polycystic ovary syndrome and related intermediary phenotypes. J Clin Endocrinol Metab 101(4):1579–1589
Sirotkin AV, Kisova G, Brenaut P, Ovcharenko D, Grossmann R, Mlyncek M (2014) Involvement of microRNA Mir15a in control of human ovarian granulosa cell proliferation, apoptosis, steroidogenesis, and response to FSH. MicroRNA (Shariqah, United Arab Emirates) 3(1):29–36
Sirotkin AV, Ovcharenko D, Grossmann R, Laukova M, Mlynček M (2009) Identification of MicroRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol 219(2):415–420
Cai G, Ma X, Chen B, Huang Y, Liu S, Yang H, Zou W (2017) MicroRNA-145 negatively regulates cell proliferation through targeting IRS1 in isolated ovarian granulosa cells from patients with polycystic ovary syndrome. Reprod Sci 24(6):902–910. doi:10.1177/1933719116673197
Yan G, Zhang L, Fang T, Zhang Q, Wu S, Jiang Y, Sun H, Hu Y (2012) MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett 586(19):3263–3270
Sirotkin AV, Lauková M, Ovcharenko D, Brenaut P, Mlynček M (2010) Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol 223(1):49–56
Hossain MM, Cao M, Wang Q, Kim JY, Schellander K, Tesfaye D, Tsang BK (2013) Altered expression of miRNAs in a dihydrotestosterone-induced rat PCOS model. J Ovar Res 6(1):36
Gebremedhn S, Salilew-Wondim D, Ahmad I, Sahadevan S, Hossain MM, Hoelker M, Rings F, Neuhoff C, Tholen E, Looft C (2015) MicroRNA expression profile in bovine granulosa cells of preovulatory dominant and subordinate follicles during the late follicular phase of the estrous cycle. PLoS One 10(5):e0125912
Gebremedhn S, Salilew-Wondim D, Hoelker M, Rings F, Neuhoff C, Tholen E, Schellander K, Tesfaye D (2016) MicroRNA-183-96-182 cluster regulates bovine granulosa cell proliferation and cell cycle transition by coordinately targeting FOXO1 1. Biol Reprod 94(6):127, 121-111
Eshre, TR, Group A-SPCW (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Ferrero H, Delgado-Rosas F, Garcia-Pascual CM, Monterde M, Zimmermann RC, Simón C, Pellicer A, Gómez R (2012) Efficiency and purity provided by the existing methods for the isolation of luteinized granulosa cells: a comparative study. Hum Reprod 27(6):1781–1789
Mohammadi-Yeganeh S, Paryan M, Samiee SM, Soleimani M, Arefian E, Azadmanesh K, Mostafavi E, Mahdian R, Karimipoor M (2013) Development of a robust, low cost stem-loop real-time quantification PCR technique for miRNA expression analysis. Mol Biol Rep 40(5):3665–3674
Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, Fei M, Sun S (2010) Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem 56(12):1830–1838
Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S (2011) Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate the existence of myocardial damage. Circ Cardiovasc Genet 4(4):446–454. doi:10.1161/CIRCGENETICS.110.958975
Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, Xing Q, Jin L, He L, Wu L (2013) Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab 98(7):3068–3079
Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG (2015) DIANA-miRPath v3. 0: deciphering microRNA function with experimental support. Nucleic Acids Res 43(W1):W460–W466. doi:10.1093/nar/gkv403
Lei L, Jin S, Gonzalez G, Behringer RR, Woodruff TK (2010) The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol 315(1):63–73
Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R, Han DY, Zhu H, Agno JE, Gunaratne PH, DeMayo FJ (2008) Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol 22(10):2336–2352
Chen Y-H, Heneidi S, Lee J-M, Layman LC, Stepp DW, Gamboa GM, Chen B-S, Chazenbalk G, Azziz R (2013) miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 62(7):2278–2286. doi:10.2337/db12-0963
Murri M, Insenser M, Fernández-Durán E, San-Millán JL, Escobar-Morreale HF (2013) Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J Clin Endocrinol Metab 98(11):E1835–E1844
Jiang L, Huang J, Li L, Chen Y, Chen X, Zhao X, Yang D (2015) MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J Clin Endocrinol Metab 100(5):E729–E738
Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee H-J, Barad D, Gleicher N, Hammes SR (2014) Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci 111(8):3008–3013
Schauer S, Sontakke S, Watson E, Esteves C, Donadeu FX (2013) Involvement of miRNAs in equine follicle development. Reproduction 146(3):273–282
Eisenberg I, Nahmias N, Persky MN, Greenfield C, Goldman-Wohl D, Hurwitz A, Haimov-Kochman R, Yagel S, Imbar T (2017) Elevated circulating micro-ribonucleic acid (miRNA)-200b and miRNA-429 levels in anovulatory women. Fertil Steril 107(1):269–275
Rodgers RJ, Irving-Rodgers HF (2010) Formation of the ovarian follicular antrum and follicular fluid 1. Biol Reprod 82(6):1021–1029
Knight PG, Glister C (2006) TGF-β superfamily members and ovarian follicle development. Reproduction 132(2):191–206
Christian M, Lam EW, Wilson MS, Brosens JJ (2011) FOXO transcription factors and their role in disorders of the female reproductive tract. Curr Drug Targets 12(9):1291–1302
Acknowledgements
This work was financially supported by the Research Deputy of Tehran University of Medical Sciences (Grant number: 92-02-30-22140).
Author information
Authors and Affiliations
Contributions
MN: protocol development, data collection, data analysis, and manuscript writing; SN: data collection and data analysis; AA: project development and data collection; EA: protocol development and data analysis; RM: protocol development and data analysis; EA: data collection and data analysis; MSN: manu writing; FA: project development, data analysis, and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors of the current paper declare no conflict of interest regarding to all time periods of working—neither financial support nor personal relationship. Mohammad Naji declares that he has no conflict of interest. Saeid nekoonam declares that he has no conflict of interest. Ashraf Aleyasin declares that she has no conflict of interest. Ehsan Arefian declares that he has no conflict of interest. Reza Mahdian declares that he has no conflict of interest. Elham Azizi declares that she has no conflict of interest. Maryam Shabani Nashtaei declares that she has no conflict of interest. Fardin Amidi declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Naji, M., Nekoonam, S., Aleyasin, A. et al. Expression of miR-15a, miR-145, and miR-182 in granulosa-lutein cells, follicular fluid, and serum of women with polycystic ovary syndrome (PCOS). Arch Gynecol Obstet 297, 221–231 (2018). https://doi.org/10.1007/s00404-017-4570-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4570-y