Abstract
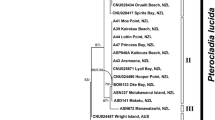
Due to the morphological variability, the identification of moss species can be difficult when the plant grows in submerged environments. The taxonomic status of an aquatic moss found in lakes of the Sôya Coast region, East Antarctica, had been controversial, and then, it was investigated by molecular phylogenetic and haplotype network analysis of two chloroplast regions (rps4 and trnL-F) and/or the nuclear ribosomal ITS region. Based on the results of the analyses, the moss was assigned to the genus Leptobryum and determined to be conspecific with Leptobryum wilsonii (Mitt.) Broth. described from South America. Almost no genetic variation was observed between all samples from Antarctic lakes and some samples of L. wilsonii from Chile. Molecular and geohistorical evidence suggests that immigration of L. wilsonii into Antarctic lakes took place during the Holocene via long-distance dispersal from South America. This study gives a clear example of the widespread assumption that most of the Antarctic moss species are post-glacial immigrants.




Similar content being viewed by others
References
Arts T (1995) Pohlia integra (Card.) Shaw a neglected species, recorded from South Africa and South America. J Bryol 18:791–796
Arts T (2001) The moss genus Leptobryum and the identity of Pohlia integra. J Bryol 23:325–330
Bradbury SM (2006) Response of the post-fire bryophyte community to salvage logging in boreal mixedwood forests of northeastern Alberta, Canada. For Ecol Manag 234:313–322
Brotherus VF (1924) Musci (Laubmoose). In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol 10, 2nd edn. pp 143–478
Churchill SP, Griffin D III, Munõz J (2000) A checklist of the mosses of the tropical Andean countries. Ruizia 17:1–203
Clement M, Posada D, Crandall K (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1660
Convey P, Gibson JAE, Hillenbrand CD, Hodgson DA, Pugh PJA, Smellie JL, Stevens MI (2008) Antarctic terrestrial life—challenging the history of the frozen continent? Biol Rev 83:103–117
Cox CJ, Goffinet B, Newton AE, Shaw AJ, Hedderson TAJ (2000) Phylogenetic relationships among the diplolepideous-alternate mosses (Bryidae) inferred from nuclear and chloroplast DNA sequences. Bryologist 103:224–241
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Frey W, Stech M, Meißner K (1999) Chloroplast DNA-relationship in palaeoaustral Lopidium concinnum (Hypopterygiaceae, Musci). An example of stenoevolution in mosses. Studies in austral temperate rain forest bryophytes 2. Plant Syst Evol 218:67–75
Frey W, Pfeiffer T, Stech M (2010) Geomolecular divergence patterns of Gondwanan and Palaeoaustral bryophytes—an overview. Studies in austral temperate rain forest bryophytes 34. Nova Hedwigia 91:317–348
Goffinet B, Cox CJ, Shaw AJ, Hedderson TAJ (2001) The bryophyta (mosses): systematic and evolutionary inferences from an rps4 gene (cpDNA) phylogeny. Ann Bot 87:191–208
Guerra J, Jiménez-Martínez JF, Cano MJ, Jiménez-Fernández JA (2011) A contribution to the phylogenetic study of Mielichhoferiaceae-Mniaceae (Bryophyta) based on molecular sequence data. Nova Hedwigia 93:47–56
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Imura S, Kanda H (1986) The gemmae of the mosses collected from the Syowa Station area, Antarctica. Mem Natl Inst Polar Res Spec Issue 44:241–246
Imura S, Higuchi M, Kanda H, Iwatsuki Z (1992) Culture of rhizoidal tubers on an aquatic moss in the lakes near the Syowa station area, Antarctica. Polar Biosci 5:177–179
Imura S, Bando T, Saito S, Seto K, Kanda H (1999) Benthic moss pillars in Antarctic lakes. Polar Biol 22:137–140
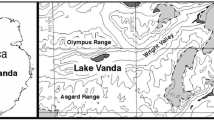
Imura S, Bando T, Seto K, Ohtani S, Kudoh S, Kanda H (2003) Distribution of aquatic mosses in the Sôya Coast region, East Antarctica. Polar Biosci 16:1–10
Iwasa T, Bando T, Nakamura T, Imura S (2000) The environmental changes presumed by AMS 14C ages of algal sediments in Antarctic lakes, near the Syowa Station. Sum Res Using AMS Nagoya Univ 11:74–80 (in Japanese with English Abstract)
Kanda H, Iwatsuki Z (1989) Two aquatic mosses in the lakes near Syowa Station, Continental Antarctica. Hikobia 10:293–297
Lewis Smith RI (1984) Colonization and recovery by cryptogams following recent volcanic activity on Deception Island, South Shetland Islands. Br Antarct Surv Bull 62:25–51
Li SP, Ochyra R, Wu PC, Seppelt RD, Cai MH, Wang HY, Li CS (2009) Drepanocladus longifolius (Amblystegiaceae), an addition to the moss flora of King George Island, South Shetland Islands, with a review of Antarctic benthic mosses. Polar Biol 32:1415–1425
Lodge E (1959) Effects of certain cultivation treatments on the morphology of some British species of Drepanocladus. J Linn Soc Lond Bot 56:218–224
Matsumoto GI, Komori K, Enomoto A, Imura S, Takemura T, Ohyama Y, Kanda H (2006) Environmental changes in Syowa Station area of Antarctica during the last 2300 years inferred from organic components in lake sediment cores. Polar Biosci 19:51–62
Matsumoto GI, Tani Y, Seto K, Tazawa T, Yamamuro M, Watanabe T, Nakamura T, Takemura T, Imura S, Kanda H (2010) Holocene paleolimnological changes in Lake Skallen Oike in the Syowa Station area of Antarctica inferred from organic components in a sediment core (Sk4C-02). J Paleolimnol 44:677–693
Murray MG, Thompson WF (1980) Rapid isolation of high-molecular-weight plant DNA. Nucleic Acids Res 8:4321–4325
Nadot S, Bittar G, Carter L, Lacroix R, Lejeune B (1995) A phylogenetic analysis of monocotyledons based on the chloroplast gene rps4, using parsimony and a new numerical phenetics method. Mol Phylogenet Evol 4:257–282
Nakai R, Abe T, Baba T, Imura S, Kagoshima H, Kanda H, Kanekiyo A, Kohara Y, Koi A, Nakamura K, Narita T, Niki H, Yanagihara K, Naganuma T (2012a) Microflorae of aquatic moss pillars in a freshwater lake, East Antarctica, based on fatty acid and 16S rRNA gene analyses. Polar Biol 35:425–433
Nakai R, Abe T, Baba T, Imura S, Kagoshima H, Kanda H, Kohara Y, Koi A, Niki H, Yanagihara K, Naganuma T (2012b) Eukaryotic phylotypes in aquatic moss pillars inhabiting a freshwater lake in East Antarctica, based on 18S rRNA gene analysis. Polar Biol 35:1495–1504
Nakanishi S (1977) Ecological studies of the moss and lichen communities in the ice-free areas near Syowa Station, Antarctica. Antarct Rec 59:68–96
Ochi H (1979) A taxonomic review of the genus Bryum, Musci in Antarctica. Mem Natl Inst Polar Res Spec Issue 11:70–80
Ochyra R, Tyshchenko O (2006) Leptobryum pyriforme (Hedw.) Wilson. In: Blockeel T (ed) New national and regional bryophyte records, 13. J Bryol 28:151–152
Ochyra R, Lewis Smith RI, Bednarek-Ochyra H (2008) The illustrated moss flora of Antarctica. Cambridge University Press, Cambridge
Peat HJ, Clarke A, Convey P (2007) Diversity and biogeography of the Antarctic flora. J Biogeogr 34:132–146
Priddle J (1979) Morphology and adaptation of aquatic mosses in an Antarctic lake. J Bryol 10:517–529
Sabovljevic M, Frahm JP, Herbiniaux U (2005) Taxonomic value, systematic position and the origin of German populations of Isothecium holtii Kindb, based on molecular data. Lindbergia 30:107–112
Savicz-Lyubitskaya LI, Smirnova ZN (1959) New species of Bryum Hedw. from the Bunger Hills. Inf Byull Sov Antarkt Eksped 7:34–39 (in Russian)
Savicz-Lyubitskaya LI, Smirnova ZN (1960) New variety of Bryum korotkevicziae Sav.-Ljub. et Z. Smim. Inf Byull Sov Antarkt Eksped 17:25–27 (in Russian)
Savicz-Lyubitskaya LI, Smirnova ZN (1964) A deep-water member of the genus Plagiothecium Br. et Sch. in Antarctica. Inf Byull Sov Antarkt Eksped 49:33–39 (in Russian)
Seto K, Imura S, Bando T, Kanda H (2002) Paleoenvironment of Holocene recorded in Antarctic lakes. Gekkan Chikyu 24:31–36 (in Japanese)
Shaw AJ (1982) Pohlia Hedw. (Musci) in North and Central America and the West Indies. Contr Univ Mich Herb 15:219–295
Shaw AJ (1984) Character analysis, phylogeny, and classification of the moss genus Pohlia. Can J Bot 62:219–229
Shaw AJ (1985) The correlation between taxonomy and peristome structure in the Bryaceae. J Hattori Bot Lab 59:79–100
Shaw AJ, Werner O, Ros RM (2003) Intercontinental Mediterranean disjunct mosses: morphological and molecular patterns. Am J Bot 90:540–550
Souza-Chies TT, Bittar G, Nadot S, Carter L, Besin E, Lejeune B (1997) Phylogenetic analysis of Iridaceae with parsimony and distance methods using the plastid gene rps4. Plant Syst Evol 204:109–123
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tewari SD, Pant G (1996) Some moss collections from Dakshin Gangotri, Antarctica. Bryol Times 91:7
Vanderpoorten A, Devos N, Goffinet B, Hardy OJ, Shaw AJ (2008) The barriers to oceanic island radiation in bryophytes: insights from the phylogeography of the moss Grimmia montana. J Biogeogr 35:654–663
White T, Burns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols. A guide to methods and applications. Academic Press, Inc, San Diego, pp 315–322
Acknowledgments
We gratefully acknowledge Dr. Jonathan Shaw and Molly McMullen (DUKE), Dr. Tomio Yamaguchi (HIRO), and Dr. Helen Peat (AAS) for loans of herbarium specimens and Dr. Satoshi Kobayashi for providing one of the Antarctic samples. We thank Kenichi Watanabe (NIPR) for his technical assistance. This research was supported by a Grant-in-Aid for Scientific Research (No. 23247012) from JSPS. We also thank two anonymous reviewers for their constructive remarks.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kato, K., Arikawa, T., Imura, S. et al. Molecular identification and phylogeny of an aquatic moss species in Antarctic lakes. Polar Biol 36, 1557–1568 (2013). https://doi.org/10.1007/s00300-013-1373-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-013-1373-x