Abstract
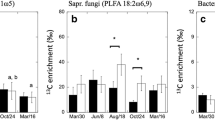
Climatic changes in Arctic regions are likely to have significant impacts on vegetation composition and physiological responses of different plant types, with implications for the regional carbon (C) cycle. Here, we explore differences in allocation and turnover of assimilated C in two Subarctic tundra communities. We used an in situ 13C pulse at mid-summer in Swedish Lapland to investigate C allocation and turnover in four contrasting tundra plant communities. We found a high rate of turnover of assimilated C in leaf tissues of Betula nana and graminoid vegetation at the height of the growing season, with a mean residence time of pulse-derived 13C of 1.1 and 0.7 days, respectively. One week after the pulse, c. 20 and 15%, respectively, of assimilated label-C remained in leaf biomass, representing most likely allocation to structural biomass. For the perennial leaf tissue of the graminoid communities, a remainder of approximately 5% of the pulse-derived C was still traceable after 1 year, whereas none was detectable in Betula foliage. The results indicate a relatively fast C turnover and small belowground allocation during the active growing season of recent assimilates in graminoid communities, with comparatively slower turnover and greater investment in belowground allocation by B. nana vegetation.





Similar content being viewed by others
References
Bowling DR, Pataki DE, Randerson JT (2008) Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol 178:24–40
Carbone MS, Trumbore SE (2007) Contribution of new photosynthetic assimilates to respiration by perennial grasses and shrubs: residence times and allocation patterns. New Phytol 176:124–135
Carbone MS, Czimczik CI, McDuffee KE, Trumbore SE (2007) Allocation and residence time of photosynthetic products in a boreal forest using a low-level C-14 pulse-chase labeling technique. Glob Change Biol 13:466–477
Chapin FS, Johnson DA, McKendrick JD (1980) Seasonal movement of nutrients in plants of differing growth form in an Alaskan tundra ecosystem—implications for herbivory. J Ecol 68:189–209
Chapin FS, Sturm M, Serreze MC, McFadden JP, Key JR, Lloyd AH, McGuire AD, Rupp TS, Lynch AH, Schimel JP, Beringer J, Chapman WL, Epstein HE, Euskirchen ES, Hinzman LD, Jia G, Ping CL, Tape KD, Thompson CDC, Walker DA, Welker JM (2005) Role of land-surface changes in Arctic summer warming. Science 310:657–660
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559
Dorrepaal E (2007) Are plant growth-form-based classifications useful in predicting northern ecosystem carbon cycling feedbacks to climate change? J Ecol 95:1167–1180
Douma JC, Van Wijk MT, Lang SI, Shaver GR (2007) The contribution of mosses to the carbon and water exchange of arctic ecosystems: quantification and relationships with system properties. Plant, Cell Environ 30:1205–1215
Euskirchen ES, McGuire AD, Chapin FS, Yi S, Thompson CC (2009) Changes in vegetation in northern Alaska under scenarios of climate change, 2003–2100: implications for climate feedbacks. Ecol Appl 19:1022–1043
Fenner N, Ostle NJ, McNamara N, Sparks T, Harmens H, Reynolds B, Freeman C (2007) Elevated CO2 effects on peatland plant community carbon dynamics and DOC production. Ecosystems 10:635–647
Gifford RM (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30:171–186
Högberg P, Högberg MN, Göttlicher SG, Betson NR, Keel SG, Metcalfe DB, Campbell C, Schindlbacher A, Hurry V, Lundmark T, Linder S, Näsholm T (2008) High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol 177:220–228
Hudson JMG, Henry GHR (2009) Increased plant biomass in a high Arctic heath community from 1981 to 2008. Ecology 90:2657–2663
Lehmeier CA, Lattanzi FA, Schaeufele R, Wild M, Schnyder H (2008) Root and shoot respiration of perennial ryegrass are supplied by the same substrate pools: assessment by dynamic C-13 labeling and compartmental analysis of tracer kinetics RID A-5609-2009. Plant Physiol 148:1148–1158
Lehmeier CA, Lattanzi FA, Gamnitzer U, Schaufele R, Schnyder H (2010a) Day-length effects on carbon stores for respiration of perennial ryegrass. New Phytol 188:719–725
Lehmeier CA, Lattanzi FA, Schaeufele R, Schnyder H (2010b) Nitrogen deficiency increases the residence time of respiratory carbon in the respiratory substrate supply system of perennial ryegrass. Plant, Cell Environ 33:76–87
McGuire AD, Anderson LG, Christensen TR, Dallimore S, Guo LD, Hayes DJ, Heimann M, Lorenson TD, Macdonald RW, Roulet N (2009) Sensitivity of the carbon cycle in the Arctic to climate change. Ecol Monogr 79:523–555
Michelsen A, Schmidt I, Jonasson S, Quarmby C, Sleep D (1996) Leaf N-15 abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non- and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia 105:53–63
Nobrega S, Grogan P (2008) Landscape and ecosystem-level controls on net carbon dioxide exchange along a natural moisture gradient in Canadian low arctic tundra. Ecosystems 11:377–396
Olsrud M, Christensen TR (2004) Carbon cycling in subarctic tundra; seasonal variation in ecosystem partitioning based on in situ 14C pulse-labelling. Soil Biol Biochem 36:245–253
Post E, Forchhammer MC, Bret-Harte MS, Callaghan TV, Christensen TR, Elberling B, Fox AD, Gilg O, Hik DS, Hoye TT, Ims RA, Jeppesen E, Klein DR, Madsen J, McGuire AD, Rysgaard S, Schindler DE, Stirling I, Tamstorf MP, Tyler NJC, van der Wal R, Welker J, Wookey PA, Schmidt NM, Aastrup P (2009) Ecological dynamics across the Arctic associated with recent climate change. Science 325:1355–1358
Roberts P, Newsham KK, Bardgett RD, Farrar JF, Jones DL (2009) Vegetation cover regulates the quantity, quality and temporal dynamics of dissolved organic carbon and nitrogen in Antarctic soils. Polar Biol 32:999–1008
Shaver GR, Chapin FS (1991) Production—biomass relationships and element cycling in contrasting arctic vegetation types. Ecol Monogr 61:1–31
Shaver GR, Giblin AE, Nadelhoffer KJ, Thieler KK, Downs MR, Laundre JA, Rastetter EB (2006) Carbon turnover in Alaskan tundra soils: effects of organic matter quality, temperature, moisture and fertilizer. J Ecol 94:740–753
Shaver GR, Street LE, Rastetter EB, Van Wijk MT, Williams M (2007) Functional convergence in regulation of net CO2 flux in heterogeneous tundra landscapes in Alaska and Sweden. J Ecol 95:802–817
Sitch S, McGuire AD, Kimball J, Gedney N, Gamon J, Engstrom R, Wolf A, Zhuang Q, Clein J, McDonald KC (2007) Assessing the carbon balance of circumpolar Arctic tundra using remote sensing and process modeling. Ecol Appl 17:213–234
Street LE, Shaver GR, Williams M, Van Wijk MT (2007) What is the relationship between changes in canopy leaf area and changes in photosynthetic CO2 flux in arctic ecosystems? J Ecol 95:139–150
Street LE, Subke JA, Sommerkorn M, Ineson P, Heinemeyer A, Williams M (2011) Turnover of recently assimilated carbon in Arctic bryophyte communities. Oecologia 167:325–337
Subke JA, Vallack HW, Magnusson T, Keel SG, Metcalfe DB, Högberg P, Ineson P (2009) Short-term dynamics of abiotic and biotic soil 13CO2 effluxes after in situ 13CO2 pulse labelling of a boreal pine forest. New Phytol 183:349–357
Tape K, Sturm M, Racine C (2006) The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Glob Change Biol 12:686–702
Trumbore S (2006) Carbon respired by terrestrial ecosystems—recent progress and challenges. Glob Change Biol 12:141–153
Van Iersel M (2003) Carbon use efficiency depends on growth respiration, maintenance respiration, and relative growth rate. A case study with lettuce. Plant, Cell Environ 26:1441–1449
van Wijk MT, Clemmensen KE, Shaver GR, Williams M, Callaghan TV, Chapin FS, Cornelissen JHC, Gough L, Hobbie SE, Jonasson S, Lee JA, Michelsen A, Press MC, Richardson SJ, Rueth H (2004) Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Global Change Biol 10:105–123
Ward SE, Bardgett RD, McNamara NP, Ostle NJ (2009) Plant functional group identity influences short-term peatland ecosystem carbon flux: evidence from a plant removal experiment. Funct Ecol 23:454–462
Waring RH, Landsberg JJ, Williams M (1998) Net primary production of forests: a constant fraction of gross primary production? Tree Physiol 18:129–134
Williams M, Rastetter EB, Shaver GR, Hobbie JE, Carpino E, Kwiatkowski BL (2001) Primary production of an arctic watershed: an uncertainty analysis. Ecol Appl 11:1800–1816
Williams M, Street LE, van Wijk MT, Shaver GR (2006) Identifying differences in carbon exchange among arctic ecosystem types. Ecosystems 9:288–304
Woodin SJ, van der Wal R, Sommerkorn M, Gornall JL (2009) Differential allocation of carbon in mosses and grasses governs ecosystem sequestration: a 13C tracer study in the high Arctic. New Phytol 184:944–949
Wookey PA, Aerts R, Bardgett RD, Baptist F, Brathen KA, Cornelissen JHC, Gough L, Hartley IP, Hopkins DW, Lavorel S, Shaver GR (2009) Ecosystem feedbacks and cascade processes: understanding their role in the responses of Arctic and alpine ecosystems to environmental change. Glob Change Biol 15:1153–1172
Acknowledgments
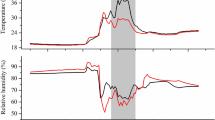
Lorna Street is thanked for dedicated support during field measurements throughout this campaign. Jon Evans of the Centre for Ecology and Hydrology is kindly acknowledged for permission to use meteorological data in Fig. 3. We would also like to thank Rafael Poyatos López, Gemma Gornall, Phil Wookey, Paul Stoy and many other members of the ABACUS consortium who supported this work with help during the York Mobile Lab deployment and plant biomass and soil harvests. The UK Natural Environment Research Council (NERC) is acknowledged for funding the experiments under the ABACUS Arctic-IPY consortium and through grant NE/E004512/1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subke, JA., Heinemeyer, A., Vallack, H.W. et al. Fast assimilate turnover revealed by in situ 13CO2 pulse-labelling in Subarctic tundra. Polar Biol 35, 1209–1219 (2012). https://doi.org/10.1007/s00300-012-1167-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-012-1167-6