Abstract
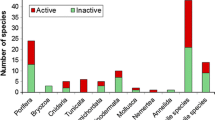
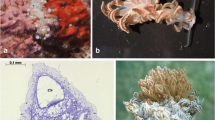
Predation and competition are important factors structuring Antarctic benthic communities and are expected to promote the production of chemical defenses. Tunicates are subject to little predation, and this is often attributed to chemical compounds, although their defensive activity has been poorly demonstrated against sympatric predators. In fact, these animals, particularly the genus Aplidium, are rich sources of bioactive metabolites. In this study, we report the natural products, distribution and ecological activity of two Aplidium ascidian species from the Weddell Sea (Antarctica). In our investigation, organic extracts obtained from external and internal tissues of specimens of A. falklandicum demonstrated to contain deterrent agents that caused repellency against the Antarctic omnivorous predator, the sea star Odontaster validus. Chemical analysis performed with Antarctic colonial ascidians Aplidium meridianum and Aplidium falklandicum allowed the purification of a group of known bioactive indole alkaloids, meridianins A-G. These isolated compounds proved to be responsible for the deterrent activity.



Similar content being viewed by others
References
Aiello A, Borrelli F, Capasso R, Fattorusso E, Luciano P, Menna M (2003) Conicamin, a novel histamine antagonist from the Mediterranean tunicate Aplidium conicum. Bioorg Med Chem Lett 13:4481–4483
Amsler CD, McClintock JB, Baker BJ (2000) Chemical defences of Antarctic marine organisms: a reevaluation of the latitudinal hypothesis. In: Davidson W, Howard-Williams C, Broady P (eds) Antarctic ecosystems: models for wider ecological understanding, Proceedings of the Seventh SCAR International Biology Symposium. NZ Natural Sciences, Christchurch, pp 158–164
Arabshahi L, Schmitz FJ (1988) Thiazole and imidazole metabolites from the ascidian Aplidium pliciferum. Tetrahedron Lett 29:1099–1102
Arntz WE, Thatje S, Gerdes D, Gili JM, Gutt J, Jacob U, Montiel A, Orejas C, Teixido N (2005) The Antarctic-Magellan connection: macrobenthos ecology on the shelf and upper slope, a progress report. Sci Mar 69(Supl 2):237–269
Arntz WE, Thatje S, Linse K, Avila C, Ballesteros M, Barnes DKA, Cope T, Cristobo FJ, De Broyer C, Gutt J, Isla E, Lopez-Gonzalez P, Montiel A, Munilla T, Espla AAR, Raupach M, Rauschert M, Rodriguez E, Teixido N (2006) Missing link in the Southern Ocean: sampling the marine benthic fauna of remote Bouvet Island. Polar Biol 29:83–96
Avila C (2006) Molluscan natural products as biological models: chemical ecology, histology and laboratory culture. In: Muller WEG (ed) Progress in molecular and subcellular biology. Subseries marine molecular biotechnology. Springer, Berlin-Heidelberg, pp 1–23
Avila C, Iken K, Fontana A, Gimino G (2000) Chemical ecology of the Antarctic nudibranch Bathydoris hodgsoni Eliot, 1907: defensive role and origin of its natural products. J Exp Biol Ecol 252:27–44
Avila C, Taboada S, Núñez-Pons L (2008) Marine Antarctic chemical ecology: what is next? Mar Ecol 29:1–70
Baker BJ, Kopitzke RW, Hamann M, McClintock JB (1993) Chemical ecology of Antarctic marine invertebrates in McMurdo Sound, Antarctica: chemical aspects. Antarct J US 28:132–133
Bakus GJ, Targett NM, Schulte B (1986) Chemical ecology of marine organisms: an overview. J Chem Ecol 12:951–987
Becerro MA, Turon X, Uriz MJ (1997) Multiple functions for secondary metabolites in encrusting marine invertebrates. J Chem Ecol 23:1527–1547
Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR (2009) Marine natural products. Nat Prod Rep 26:170–244
Carliste DB (1968) Vanadium and other metals in ascidians. Proc R Soc London Ser B 171:31–42
Carroll AR, Bowden BF, Coll JC (1993) Studies of Australian ascidians II. Novel cytotoxic iodotyrosine-based alkaloids from colonial ascidians, Aplidium sp. Aust J Chem 46:825–832
Chanas B, Pawlik JR (1995) Defenses of Caribbean sponges against predatory reef fish: II. Spicules, tissue toughness, and nutritional quality. Mar Ecol Prog Ser 127:195–211
Chanas B, Pawlik JR (1996) Does the skeleton of a sponge provide a defense against predatory reef fish? Oecologia 107:225–231
Davidson BS (1993) Ascidians: producers of amino acid derived metabolites. Chem Rev 93:1771–1791
Davis AR, Bremner JB (1999) Potential antifouling natural products from ascidians: a review. In: Fingerman M, Nagabhushanam R, Thompson M-F (eds) Marine biotechnology recent advances in marine biotechnology, Vol III. Biofilms, bioadhesion, corrosion and biofouling. Science Publishers, Enfield, pp 259–308
Davis RA, Carroll AR, Quinn RJ (2002) Lepadins F-H, new cis-decahydroquinoline alkaloids from Australian ascidian Aplidium tabascum. J Nat Prod 65:454–457
Dayton PK, Robillia GA, Paine RT, Dayton LB (1974) Biological accommodation in benthic community at McMurdo Sound Antarctica. Ecol Monogr 44:105–128
Dearborn JH (1977) Food and feeding characteristics of Antarctic asteroids and ophiuroids. In: Llano GAE (ed) Adaptations within Antarctic ecosystems. Gulf Publications Co, Houston, pp 293–326
Diyabalanage T, Amsler CD, Mcclintock JB, Baker BJ (2006) Palmerolide A, a cytotoxic macrolide from the Antarctic tunicate Synoicum Adareanum. J Am Chem Soc 128:5630–5631
Faulkner DJ (2000) Marine pharmacology. Antonie Van Leeuwenhoek 77:135–145
Fenical W (2007) Marine natural products: where we’ve been and where we’re going? 12th International Symposium on Marine Natural Products. Queenstown, New Zealand: Proceedings, 74
Garrido L, Zubía E, Ortega MJ, Salva J (2003) Haouamines A and B: a new class of alkaloids from ascidian Aplidium haouarianum. J Org Chem 68:293–299
Gili JM, Orejas C, Ros JD, López PJ, Arntz WE (2000) La vida en los fondos antárticos. Invest Cienc 290:64–74
Gompel M, Leost M, Bal de Kier Joffé E, Puricelli L, Hernández Franco L, Palermo JA, Meijer L (2004) Meridianins, a new family of protein kinase inhibitors isolated from the Ascidian Aplidium meridianum. Bioorg Med Chem Lett 14:1703–1707
Goodbody L, Gibson J (1974) The biology of Ascidia nigra (Savigny) V. Survival in populations settled at different times of the year. Biol Bull 146:217–237
Gutt J (2000) Some “driving forces” structuring communities of the sublittoral Antarctic macrobenthos. Antarct Sci 12:297–313
Gutt J, Starmans A (1998) Structure and biodiversity of megabenthos in the Weddell and Lazarev Seas (Antarctica): ecological role of physical parameters and biological interactions. Polar Biol 20:229–247
Hernández Franco L, Bal de Kier Joffé E, Puricelli L, Tatián M, Seldes AM, Palermo JA (1998) Indole alkaloids from the Tunicate Aplidium meridianum. J Nat Prod 61:1130–1132
Iken K, Avila C, Fontana A, Gavagnin M (2002) Chemical ecology and origin of defensive compounds in the Antarctic nudibranch Austrodoris kerguelenensis (Opisthobranchia: Gastropoda). Mar Biol 141:101–109
Kim J, Pordesimo EO, Toth SI, Schmitz FJ, Vanaltena I (1993) Pantherinine, a cytotoxic aromatic alkaloid, and 7-deazainosine from ascidian Aplidium pantherinum. J Nat Prod 56:1813–1816
Koplovitz G, McClintock JB, Amsler CD, Baker BJ (2009) Palatability and anti-predatory chemical defenses in a suite of ascidians from the Western Antarctic Peninsula. Aquat Biol 7:81–92
Lambert G, Lambert CC (1987) Spicule formation in the solitary ascidian, Herdmania momus. J Morphol 192:145–159
Lebar MD, Heimbegner JL, Baker BJ (2007) Cold-water marine natural products. Nat Prod Rep 24:774–797
Lindquist N, Hay ME (1996) Palatability and chemical defense of marine invertebrate larvae. Ecol Monogr 66:431–450
Lindquist N, Hay ME, Fenical W (1992) Defense of ascidians and their conspicuous larvae: adult vs larval chemical defenses. Ecol Monogr 62:547–568
López-Legentil S, Turón X, Schupp P (2006) Chemical and physical defenses against predators in Cystodytes (Ascidiacea). J Exp Mar Biol Ecol 332:27–36
McClintock JB (1989) Toxicity of shallow-water Antarctic echinoderms. Polar Biol 9:461–465
McClintock JB (1994) Trophic biology of Antarctic echinoderms. Mar Ecol Prog Ser 111:191–202
McClintock JB, Baker BJ (1997) Palatability and chemical defense of eggs, embryos and larvae of shallow-water Antarctic marine invertebrates. Mar Ecol Prog Ser 154:121–131
McClintock JB, Baker BJ (2001) Marine chemical ecology. CRC Marine Science Series Press, Boca Raton
McClintock JB, Heine J, Slattery M, Weston J (1991) Biochemical and energetic composition, population biology, and chemical defense of the Antarctic ascidian Cnemidocarpa verrucosa Lesson. J Exp Mar Biol Ecol 147:163–175
McClintock JB, Slattery M, Heine L, Weston J (1992) Chemical ecology of the Antarctic spongivorous seastar Perknaster fuscus. Antarct J US 27:129–130
McClintock JB, Baker BJ, Slattery M, Hamann M, Kopitzke R, Heine J (1994) Chemotactic tube-foot responses of a spongivorous sea star Perknaster fuscus to organic extracts from Antarctic sponges. J Chem Ecol 20:859–870
McClintock JB, Amsler MO, Amsler CD, Southworth KJ, Petrie C, Baker BJ (2004) Biochemical composition, energy content and chemical antifeedant and antifoulant defenses of the colonial Antarctic ascidian Distaplia cylindrica. Mar Biol 145:885–894
Mccoy MC, Faulkner DJ (2001) Uoamines A and B, piperidine alkaloids from the ascidian Aplidium uouo. J Nat Prod 64:1087–1089
Millar RH (1971) The biology of ascidians. Adv Mar Biol 9:1–100
Munro MHG, Blunt JW (2009) MarineLit. University of Canterbury
Murray L, Lim TK, Currie G, Capon RJ (1995) Aplidites (A-G): macrocyclic orthonitrites from an Australian tunicate, Aplidium sp. Aust J Chem 48:1253–1266
Parry DL (1984) Chemical properties of the test ascidians in relation to predation. Mar Ecol Prog Ser 17:279–282
Paul VJ (1992) Ecological roles of marine natural products. Comstock Publications Association, Ithaca, New York
Paul VJ, Lindquist N, Fenical W (1990) Chemical defenses of the tropical ascidian Atapozoa sp. and its nudibranch predators Nembrotha spp. Mar Ecol Prog Ser 59:109–118
Paul VJ, Puglisi MP, Ritson-Williams R (2008) Marine chemical ecology. Nat Prod Rep 25:662–695
Pawlik JR (1993) Marine invertebrate chemical defenses. Chem Rev 93:1911–1922
Pawlik JR, Chanas RT, Toonen RT, Fenical W (1995) Defenses of Caribean sponges against predatory reef fish. I. Chemical deterrency. Mar Ecol Prog Ser 127:183–194
Peters KJ, Amsler CD, McClintock JB, van Soest RWM, Baker BJ (2009) Palatability and chemical defenses of sponges from the western Antarctic Peninsula. Mar Ecol Prog Ser 385:179–187
Pisut DP, Pawlik JR (2002) Anti-predatory chemical defenses of ascidians: secondary metabolites or inorganic acids? J Exp Mar Biol Ecol 270:203–214
Primo C, Vázquez E (2007) Zoogeography of the Antarctic ascidian fauna in relation to the Sub-Antarctic and South America. Antarct Sci 19:321–336
Puglisi MP, Paul VJ, Biggs J, Slattery M (2002) Co-occurrence of chemical and structural defenses in the gorgonian corals of Guam. Mar Ecol Prog Ser 239:105–114
Ramos-Esplá AA, Cárcel JA, Varela M (2005) Zoogeographical relationships of the littoral ascidiofauna around the Antarctic Peninsula, in the Scotia Arc and in the Magellan region. Sci Mar 69(Suppl 2):215–223
Reyes F, Fernández R, Rodríguez A, Francesch A, Taboada S, Avila C (2008) Aplicyanins A-F, new cytotoxic bromoindole derivates from the marine tunicate Aplydium cyaneum. Tetrahedron 64:5119–5123
Rhoades DF (1979) Evolution of plant chemical defenses against herbivores. In: Rosenthal GA (ed) Herbivores: their interaction with secondary plant metabolites. Academic Press, Orlando, pp 4–55
Sahade R, Demarchi M, Chiappero M, Tatian M, Gardenal N (2003) Genetic differentiation between populations of the ascidian Aplidium falklandicum from South Georgia and South Orkney Islands. In: Thatje S, Calcagno J, Arntz W (eds) Interactions between Magellan region and the Antarctic—Antarctic benthic deep-sea biodiversity. Extended Abstracts of the IBMANT/ANDEEP International Symposium and Workshop, pp 95–96
Seldes AM, Rodríguez Brasco MF, Hernández Franco L, Palermo JA (2007) Identification of two meridianins from the crude extract of the tunicate Aplidium meridianun by tandem mass spectometry. Nat Prod Res 21:555–563
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of stadistics in biological research. Freeman WH and Co, New York
Stoecker D (1980a) Chemical defenses of ascidians against predators. Ecology 61:1327–1334
Stoecker D (1980b) Relationships between chemical defense an ecology in benthic ascidians. Mar Ecol Prog Ser 3:257–265
Taboada S, García-Fernández LF, Bueno S, Vazquez J, Cuevas C Avila C (2010) Antitumoral activity in Antarctic and Sub-Antarctic benthic organisms. Antarct Sci (Accepted)
Tarjuelo I, López-Legentil S, Codina M, Turón X (2002) Defense mechanisms of adults and larvae of marine invertebrates: patterns of toxicity and palatability in colonial ascidians. Mar Ecol Prog Ser 235:103–115
Tatián M (1999) Diversidad, variabilidad y alimentación de ascidias (Tunicata, Ascidiacea) de la Isla 25 de Mayo. Dissertation. Universidad de Córdoba, Argentina
Tatián M, Sahade RJ, Doucet ME, Esnal GB (1998) Ascidians (Tunicata, Ascidiacea) of Potter Cove South Shetland Islands, Antarctica. Antarct Sci 10:147–152
Tatián M, Antacli JC, Sahade RJ (2005) Ascidians (Tunicata, Ascidiacea): species distribution along the Scotia Arc. Sci Mar 69(Suppl 2):205–214
Varela M (2007) Contribución al conocimiento de las ascidias coloniales (Chordata: Tunicata) de la Antártida Occidental y Región Magallánica. Dissertation. Universitat d’Alacant, Spain
Vervoort HC, Pawlik JR, Fenical W (1998) Chemical defense of the Caribean ascidian Didemnum conchyliatum. Mar Ecol Prog Ser 164:221–228
Young CM, Bingham BL (1987) Chemical defense and aposematic coloration in larvae of the ascidian Ecteinascidia turbinata. Mar Biol 96:539–544
Zubía E, Ortega MJ, Salvá J (2005) Natural products chemistry in marine ascidians of the genus Aplidium. Mini-Rev Org Chem 2:389–399
Acknowledgments
We wish to thank W. Arntz and the R/V Polarstern crew for their help and support during the ANT XXI/2 cruise, as well as the BIO Hespérides and the BAE “Gabriel de Castilla” teams during the ECOQUIM cruise. Funding was provided by the Ministry of Science and Education of Spain through the ECOQUIM Projects (REN2003-00545, REN2002-12006E ANT and CGL2004-03356/ANT). Also thanks are due to S. Taboada for his laboratory support, as well as in the field work. We are thankful to J. Vázquez, B. Figuerola and D. Melck for helping in the laboratory and in the preparation of the experiments and to F. J. Cristobo, J. L. Moya and M. Ballesteros and the Bentart team for their help in collecting the sea stars in Deception Island during the ECOQUIM 2006 cruise. Thanks are also due to “Servizo de Apoio a Investigación (SAI-UDC)” for instrumental support. L. Núñez-Pons was consecutively supported by PharmaMar S.A., an I3P (CSIC) grant and a FPU Fellowship from the Ministry of Education (MEC) during this study. Finally, we wish to thank the reviewers for their helpful comments and the Serveis Lingüístics of the UB for reviewing our English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Núñez-Pons, L., Forestieri, R., Nieto, R.M. et al. Chemical defenses of tunicates of the genus Aplidium from the Weddell Sea (Antarctica). Polar Biol 33, 1319–1329 (2010). https://doi.org/10.1007/s00300-010-0819-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0819-7