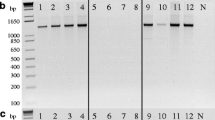
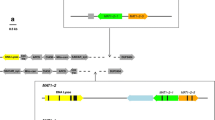
Abstract.
Phaeosphaeria avenaria, one of the causal agents of stagonospora leaf blotch diseases in cereals, is composed of two subspecies, P. avenaria f. sp. triticea (Pat) and P. avenaria f. sp. avenaria (Paa). The Pat subspecies was grouped into Pat1–Pat3, based on restriction fragment length polymorphism (RFLP) and ribosomal DNA (rDNA) internal transcribed spacer (ITS) sequences in previous studies. Mating-type genes and their potential use in phylogeny and molecular classification were studied by DNA hybridization and PCR amplification. The majority of Pat1 isolates reported to be homothallic and producing sexual reproduction structures on cultural media had only the MAT1-1 gene. Minor sequence variations were found in the conserved region of MAT1-1 gene in Pat1 isolates. However, both mating-type genes, MAT1-1 and MAT1-2, were identified in P. avenaria isolates represented by ATCC12277 from oats (Paa) and the Pat2 isolates from foxtail barley (Hordeum jubatum L.). Cluster analyses based on mating-type gene conserved regions revealed that cereal Phaeosphaeria is not phylogenetically closely related to other ascomycetes, including Mycosphaerella graminicola (anamorph Septoria tritici). The sequence diversity of mating-type genes in Pat and Paa supports our previous phylogenetic relationship and molecular classification based on RFLP fingerprinting and rDNA ITS sequences.






Similar content being viewed by others
References
Arseniuk E, Czembor HJ, Sowa W, Krysiak H (1989) Preliminary studies of Septoria spp on triticale in Poland. In: Fried PM (ed) Septoria of cereals: proceedings. Swiss Federal Research Station for Agronomy, Zurich, pp 16–18
Beck JJ, Ligon JM (1995) Polymerase chain reaction assays for the detection of Stagonospora nodorum and Septoria tritici in wheat. Phytopathology 85:319–324
Beck JJ, Ligon JM, Etienne L, Binder A (1996) Detection of crop fungal pathogens by polymerase chain reaction technology. In: Marshall G (ed) Diagnostics in crop production. The British Crop Protection Council, Farnham, pp 111–118
Bennett RS, Yun SH, Lee TY, Turgeon BG, Cunfer B, Arseniuk E, Bergstrom GC (1999) Mating type-specific PCR primers for Stagonospora nodorum field studies. In: Ginkel M van, McNab MA, Krupinsky J (eds) Septoria and Stagonospora diseases of cereals: a compilation of global research. CIMMYT, Mexico, D.F., pp 90–92
Câmara MPS, Palm ME, Berkum P van, O'Neill NR (2002) Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia 94:630–640
Caten CE, Newton AC (2000) Variation in cultural characteristics, pathogenicity, vegetative compatibility and electrophoretic karyotype within field populations of Stagonospora nodorum. Plant Pathol 49:219–226
Clark RV, Gourley CO, Johnston HW, Piening LJ, Pelletier G, Santerre J, Genereux H (1975) Oat yield losses from Septoria leaf blotch at four locations in eastern Canada. Can Plant Dis Surv 55:36–43
Coppin E, Debuchy R, Arnaise S, Picard M (1997) Mating-types and sexual development in filamentous ascomycetes. Microbiol Mol Biol Rev 61:411–428
Cunfer BM, Ueng PP (1999) Taxonomy and identification of Septoria and Stagonospora species on small-grain cereals. Annu Rev Phytopathol 37:267–284
Czembor PC, Arseniuk E (2000) Segregation and recombination of PCR based markers in sexual progeny of Phaeosphaeria species. Mycol Res 104:919–926
Dai Q, Arseniuk E, Cunfer BM, Cui K, Ueng PP (2001) Segregation, aggressiveness and sexuality in Phaeosphaeria nodorum. Phytopathology 91:S20
Glass NL, Kuldau GA (1992) Mating type and vegetative incompatibility in filamentous ascomycetes. Annu Rev Phytopathol 30:201–224
Glass NL, Smith ML (1994) Structure and function of a mating-type gene from the homothallic species Neurospora africana. Mol Gen Genet 244:401–409
Glass NL, Metzenberg RL, Raju NB (1990) Homothallic Sordariaceae from nature: the absence of strains containing only the α mating type sequence. Exp Mycol 14:273–289
Goodwin SB, Zismann VL (2001) Phylogenetic analyses of the ITS region of ribosomal DNA reveal that Septoria passerinii from barley is closely related to the wheat pathogen Mycosphaerella graminicola. Mycologia 93:934–946
Goodwin SB, Dunkle LD, Zismann VL (2001) Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology 91:648–658
Halama P (2002) Mating relationships between isolates of Phaeosphaeria nodorum, (anamorph Stagonospora nodorum) from geographical locations. Eur J Plant Pathol 108:593–596
Halama P, Lacoste L (1991) Déterminisme de la reproduction sexuée de Phaeosphaeria (Leptosphaeria) nodorum, agent de la septoriose du blé. I. Hétérothallisme et rôle des microspores. Can J Bot 69:95–99
Halama P, Skajennikoff M, Dehorter B (1999) Tetra analysis of mating type, mutations, esterase and aggressiveness in Phaeosphaeria nodorum. Mycol Res 103:43–49
Hosford RM Jr, Hogenson RO, Huguelet JE, Kiesling RL (1969) Studies of Leptosphaeria avenaria f. sp. triticea on wheat in North Dakota. Plant Dis Rep 53:378–381
Johnson T (1947) A form of Leptosphaeria avenaria on wheat in Canada. Can J Res Sect C 25:259–270
Keller SM, McDermott JM, Pettway RE, Wolfe MS, McDonald BA (1997) Gene flow and sexual reproduction in the wheat glume blotch pathogen Phaeosphaeria nodorum (anamorph Stagonospora nodorum). Phytopathology 87:353–358
Krüger J, Hoffmann GM (1978) Differenzierung von Septoria nodorum Berk. und Septoria avenae Frank f. sp. triticea T. Johnson. Z Pflanzenkr Pflanzenschutz 85:645–650
Luz WC da, Bergstrom GC (1985) Septoria avenae spot as an additional component of the fungal leaf spot syndrome of spring wheat in New York. Plant Dis 69:724–725
Mäkelä K (1977) Septoria and Selenophoma species on Gramineae in Finland. Ann Agric Fenn 16:256–276
McDonald BA, Zhan J, Yarden O, Hogan K, Garton J, Pettway RE (1999) The population genetics of Mycosphaerella graminiola and Phaeosphaeria nodorum. In: Lucas JA, Bowyer P, Anderson HM (eds) Septoria on cereals: a study of pathosystems. CAB, Wallingford, pp 44–69
Metzenberg RL, Glass NL (1990) Mating type and mating strategies in Neurospora. BioEssays 12:53–59
Mielke H (1975) Über die Blattfleckenkrankheit (Septoria avenae Frank) des Hafers. Mitt Biol Bundesanst Land Forstwirtsch Berlin-Dahlem 163:41–47
Pöggeler S (1999) Phylogenetic relationships between mating-type sequences from homothallic and heterothallic ascomycetes. Curr Genet 36:222–231
Pöggeler S, Risch S, Kück U, Osiewacz HD (1997) Mating-type genes from the homothallic fungus Sordaria macrospora are functionally expressed in a heterothallic ascomycete. Genetics 147:567–580
Richardson MJ, Noble M (1970) Septoria species on cereals—a note to aid their identification. Plant Pathol 19:159–163
Shaw DE (1957) Studies on Leptosphaeria avenaria f. sp. triticea on cereals and grasses. Can J Bot 35:113–118
Shearer BL, Skovmand B, Wilcoxson RD (1977) Hordeum jubatum as a source of inoculum of Septoria avenae f. sp. triticea and S. passerinii. Phytopathology 67:1338–1341
Shipton WA, Boyd WJR, Rosielle AA, Shearer BL (1971) The common Septoria diseases of wheat. Bot Rev 37:231–262
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Sprague R (1950) Disease of cereals and grasses in North America. Ronald Press: New York
Staben C, Yanofsky C (1990) Neurospora crassa a mating-type region. Proc Natl Acad Sci USA 87:4917–4921
Turgeon BG (1998) Application of mating type gene technology to problems in fungal biology. Annu Rev Phytopathol 36:115–137
Turgeon BG, Yoder OC (2000) Proposed nomenclature for mating type genes of filamentous Ascomycetes. Fungal Genet Biol 31:1–5
Ueng PP, Chen W (1994) Genetic differentiation between Phaeosphaeria nodorum and P. avenaria using restriction fragment length polymorphisms. Phytopathology 84:800–806
Ueng PP, Bergstrom GC, Slay RM, Geiger EA, Shaner G, Scharen AL (1992) Restriction fragment length polymorphisms in the wheat glume blotch fungus, Phaeosphaeria nodorum. Phytopathology 82:1302–1305
Ueng PP, Cunfer BM, Alano AS, Youmans JD, Chen W (1995) Correlation between molecular and biological characters in identifying the wheat and barley biotypes of Stagonospora nodorum. Phytopathology 85:44–52
Ueng PP, Subramaniam K, Chen W, Arseniuk E, Wang L, Cheung AM, Hoffmann GM, Bergstrom GC (1998) Intraspecific genetic variation of Stagonospora avenae and its differentiation from S. nodorum. Mycol Res 102:607–614
Waalwijk C, Mendes O, Verstappen ECP, Waard MA de, Kema GHJ (2002) Isolation and characterization of the mating-type idiomorphs from the wheat septoria leaf blotch fungus Mycosphaerella graminicola. Fungal Genet Biol 35:277–286
Weber GF (1922) Septoria diseases of cereals. I. Speckled blotch of oats caused by Leptosphaeria. Phytopathology 12:449–470
Yun S, Berbee ML, Yoder OC, Turgeon BG (1999) Evolution of the fungal self-fertile reproductive life style from self-sterile ancestors. Proc Natl Acad Sci USA 96:5592–5597
Yun S, Arie T, Kaneko I, Yoder OC, Turgeon BG (2000) Molecular organization of mating type loci in heterothallic, homothallic, and asexual Gibberella/Fusarium species. Fungal Genet Biol 31:7–20
Zeiders KE, Berg CC, Sherwood RT (1984) Effect of recurrent phenotypic selection on resistance to purple leaf spot in orchardgrass. Crop Sci 24:182–185
Zhan J, Kema GHJ, Waalwijk C, McDonald BA (2002) Distribution of mating type alleles in the wheat pathogen Mycosphaerella graminicola over spatial scales from lesions to continents. Fungal Genet Biol 36:128–136
Acknowledgements.
We thank Rosemarie Hammond of the USDA–ARS for reviewing the manuscript and Z. Ye of the University of Maryland for technical support. This research is partially supported by the USDA–ARS CRIS and the US–Poland Maria Sklodowska–Curie Joint Fund Projects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Kück
Rights and permissions
About this article
Cite this article
Ueng, P.P., Dai, Q., Cui, Kr. et al. Sequence diversity of mating-type genes in Phaeosphaeria avenaria . Curr Genet 43, 121–130 (2003). https://doi.org/10.1007/s00294-003-0377-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-003-0377-4