Abstract
Purpose
It has been supposed that cardiac toxicity of doxorubicin is due to its production of free radicals and inflammatory cytokines. Dapsone, an antibiotic drug which is the principal in a multidrug regimen for the treatment of leprosy, is a sulfone with anti-inflammatory and antioxidant immunosuppressive properties. Therefore, we designed this study to investigate the possible effects of dapsone on doxorubicin-induced cardiotoxicity.
Methods
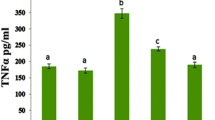
Male rats were administrated doxorubicin (2.5 mg/kg) and dapsone (1, 3, 10 mg/kg) intraperitoneally six times in 2 weeks. Then electrocardiographic (ECG) parameters (QRS complexes, RR and QT intervals) alternation, papillary muscle contraction and excitation, and histopathological changes were assessed. Also, the heart tissue levels of malondialdehyde (MDA) as oxidant factor and superoxide dismutase (SOD) as antioxidant enzyme, tumor necrosis factor-alpha (TNF-α) and serum level of CK-MB were analyzed.
Results
Administration of dapsone with doxorubicin significantly reversed alterations induced by doxorubicin in serum levels of CK-MB, ECG parameters, papillary muscle contractility and excitation. Furthermore, the measurement of MDA, SOD and TNF-α tissue level indicated that dapsone significantly reduced oxidative stress and inflammation. These findings were consistent with histopathological analysis.
Conclusion
Dapsone exerts cardioprotective effects on doxorubicin-induced cardiotoxicity through its anti-inflammatory and antioxidant mechanism.





Similar content being viewed by others
References
Wonders KY, Hydock DS, Schneider CM, Hayward R (2008) Acute exercise protects against doxorubicin cardiotoxicity. Integr Cancer Ther 7(3):147–154
Gandhi H, Patel VB, Mistry N, Patni N, Nandania J, Balaraman R (2013) Doxorubicin mediated cardiotoxicity in rats: protective role of felodipine on cardiac indices. Environ Toxicol Pharmacol 36(3):787–795
Guo R, Hua Y, Ren J, Bornfeldt KE, Nair S (2018) Cardiomyocyte-specific disruption of Cathepsin K protects against doxorubicin-induced cardiotoxicity. Cell Death Dis 9(6):692
Kim SH, Kim K-J, Kim J-H, Kwak J-H, Song H, Cho JY, Hwang DY, Kim KS, Jung Y-S (2017) Comparision of doxorubicin-induced cardiotoxicity in the ICR mice of different sources. Lab Anim Res 33(2):165–170
Khalilzadeh M, Abdollahi A, Abdolahi F, Abdolghaffari AH, Dehpour AR, Jazaeri F (2018) Protective effects of magnesium sulfate against doxorubicin induced cardiotoxicity in rats. Life Sci 207:436–441
Pecoraro M, Ciccarelli M, Fiordelisi A, Iaccarino G, Pinto A, Popolo A (2018) Diazoxide improves mitochondrial connexin 43 expression in a mouse model of doxorubicin-induced cardiotoxicity. Int J Mol Sci 19(3):757
Angsutararux P, Luanpitpong S, Issaragrisil S (2015) Chemotherapy-induced cardiotoxicity: overview of the roles of oxidative stress. Oxid Med Cell Longev. https://doi.org/10.1155/2015/795602
dos Santos JM, Alfredo TM, Antunes KÁ, da Cunha JDSM, Costa EMA, Lima ES, Silva DB, Carollo CA, Schmitz WO, Boleti APDA (2018) Guazuma ulmifolia Lam. Decreases Oxidative Stress in Blood Cells and Prevents Doxorubicin-Induced Cardiotoxicity. Oxid Med Cell Longev. DOI: 10.1155/2018/2935051.
Pecoraro M, Del Pizzo M, Marzocco S, Sorrentino R, Ciccarelli M, Iaccarino G, Pinto A, Popolo A (2016) Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol Appl Pharmacol 293:44–52
Wozel G, Blasum C (2014) Dapsone in dermatology and beyond. Arch Dermatol Res 306(2):103–124
Kwon M-J, Joo H-G (2018) Dapsone modulates lipopolysaccharide-activated bone marrow cells by inducing cell death and down-regulating tumor necrosis factor-α production. J Vet Sci 19(6):744–749
Ríos C, Farfán-Briseño AC, Manjarrez-Marmolejo J, Franco-Pérez J, Méndez-Armenta M, Nava-Ruiz C, Caballero-Chacón S, Ruiz-Diaz A, Baron-Flores V, Díaz-Ruiz A (2019) Efficacy of dapsone administered alone or in combination with diazepam to inhibit status epilepticus in rats. Brain Res 1708:181–187
Ríos C, Orozco-Suarez S, Salgado-Ceballos H, Mendez-Armenta M, Nava-Ruiz C, Santander I, Barón-Flores V, Caram-Salas N, Diaz-Ruiz A (2015) Anti-apoptotic effects of dapsone after spinal cord injury in rats. Neurochem Res 40(6):1243–1251
Zhan R, Zhao M, Zhou T, Chen Y, Yu W, Zhao L, Zhang T, Wang H, Yang H, Jin Y (2018) Dapsone protects brain microvascular integrity from high-fat diet induced LDL oxidation. Cell Death Dis 9(6):683
Sumitra M, Manikandan P, Rao KVK, Nayeem M, Manohar BM, Puvanakrishnan R (2004) Cardiorespiratory effects of diazepam–ketamine, xylazine–ketamine and thiopentone anesthesia in male Wistar rats-a comparative analysis. Life Sci 75(15):1887–1896
Arini PD, Liberczuk S, Mendieta JG, Santa María M, Bertrán GC (2018) Electrocardiogram delineation in a Wistar rat experimental model. Comput Math Methods Med. https://doi.org/10.1155/2018/2185378
Yarmohmmadi F, Rahimi N, Faghir-Ghanesefat H, Javadian N, Abdollahi A, Pasalar P, Jazayeri F, Ejtemaeemehr S, Dehpour AR (2017) Protective effects of agmatine on doxorubicin-induced chronic cardiotoxicity in rat. Eur J Pharmacol 796:39–44
Kelishomi RB, Ejtemaeemehr S, Tavangar SM, Rahimian R, Mobarakeh JI, Dehpour AR (2008) Morphine is protective against doxorubicin-induced cardiotoxicity in rat. Toxicology 243(1–2):96–104
Muthuraman A, Singh N, Jaggi AS (2011) Protective effect of Acorus calamus L. in rat model of vincristine induced painful neuropathy: an evidence of anti-inflammatory and anti-oxidative activity. Food Chem Toxicol 49(10):2557–2563
Piegari E, Russo R, Cappetta D, Esposito G, Urbanek K, Dell'Aversana C, Altucci L, Berrino L, Rossi F, De Angelis A (2016) MicroRNA-34a regulates doxorubicin-induced cardiotoxicity in rat. Oncotarget 7(38):62312
Volkova M, Russell R (2011) Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev 7(4):214–220
Giampieri F, Alvarez-Suarez JM, Gasparrini M, Forbes-Hernandez TY, Afrin S, Bompadre S, Rubini C, Zizzi A, Astolfi P, Santos-Buelga C (2016) Strawberry consumption alleviates doxorubicin-induced toxicity by suppressing oxidative stress. Food Chem Toxicol 94:128–137
Tahover E, Patil YP, Gabizon AA (2015) Emerging delivery systems to reduce doxorubicin cardiotoxicity and improve therapeutic index: focus on liposomes. Anticancer Drugs 26(3):241–258
Subbarao R, Ok S-H, Lee S, Kang D, Kim E-J, Kim J-Y, Sohn J-T (2018) Lipid emulsion inhibits the late apoptosis/cardiotoxicity induced by doxorubicin in rat cardiomyoblasts. Cells 7(10):144
Rašković A, Stilinović N, Kolarović J, Vasović V, Vukmirović S, Mikov M (2011) The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules 16(10):8601–8613
Zhang T, Tian X, Wang Q, Tong Y, Wang H, Li Z, Li L, Zhou T, Zhan R, Zhao L (2015) Surgical stress induced depressive and anxiety like behavior are improved by dapsone via modulating NADPH oxidase level. Neurosci Lett 585:103–108
Cho SC, Park MC, Keam B, Choi JM, Cho Y, Hyun S, Park SC, Lee J (2010) DDS, 4, 4′-diaminodiphenylsulfone, extends organismic lifespan. Proc Natl Acad Sci 107(45):19326–19331
Suda T, Suzuki Y, Matsui T, Inoue T, Niide O, Yoshimaru T, Suzuki H, Ra C, Ochiai T (2005) Dapsone suppresses human neutrophil superoxide production and elastase release in a calcium-dependent manner. Br J Dermatol 152(5):887–895
Horacek J, Jakl M, Horackova J, Pudil R, Jebavy L, Maly J (2009) Assessment of anthracycline-induced cardiotoxicity with electrocardiography. Exp Oncol 31(2):115–117
Antoniou C-K, Dilaveris P, Manolakou P, Galanakos S, Magkas N, Gatzoulis K, Tousoulis D (2017) QT prolongation and malignant arrhythmia: how serious a problem? Eur Cardiol Rev 12(2):112
Sordillo PP, Sordillo DC, Helson L (2015) The prolonged QT Interval: role of pro-inflammatory cytokines, reactive oxygen species and the ceramide and sphingosine-1 phosphate pathways. vivo 29(6):619–636
Adlan AM (2018) Inflammation and heart rate-corrected QT interval: evidence for a potentially reversible cause of sudden death in patients with rheumatoid arthritis? J Rheumatol. https://doi.org/10.3899/jrheum.180921
Ribeiro-Rodrigues TM, Martins-Marques T, Morel S, Kwak BR, Girão H (2017) Role of connexin 43 in different forms of intercellular communication–gap junctions, extracellular vesicles and tunnelling nanotubes. J cell Sci 130(21):3619–3630
Pecoraro M, Sorrentino R, Franceschelli S, Del Pizzo M, Pinto A, Popolo A (2015) Doxorubicin-mediated cardiotoxicity: role of mitochondrial connexin 43. Cardiovasc Toxicol 15(4):366–376
Pecoraro M, Rodríguez-Sinovas A, Marzocco S, Ciccarelli M, Iaccarino G, Pinto A, Popolo A (2017) Cardiotoxic effects of short-term doxorubicin administration: involvement of connexin 43 in calcium impairment. Int J Mol Sci 18(10):2121
Oliveira MS, Melo MB, Carvalho JL, Melo IM, Lavor MS, Gomes DA, de Goes AM, Melo MM (2013) Doxorubicin cardiotoxicity and cardiac function improvement after stem cell therapy diagnosed by strain echocardiography. J Cancer Sci Therapy 5(2):052
Galal A, El Bakly WM, Al Haleem ENA, El-Demerdash E (2016) Selective A 3 adenosine receptor agonist protects against doxorubicin-induced cardiotoxicity. Cancer Chemother Pharmacol 77(2):309–322
Akolkar G, Bagchi AK, Ayyappan P, Jassal DS, Singal PK (2017) Doxorubicin-induced nitrosative stress is mitigated by vitamin C via the modulation of nitric oxide synthases. Am J Physiol Cell Physiol 312(4):C418–C427
Akolkar G, da Silva DD, Ayyappan P, Bagchi AK, Jassal DS, Salemi VMC, Irigoyen MC, De Angelis K, Singal PK (2017) Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 313(4):H795–H809
El-Aziz TAA, Mohamed RH, Pasha HF, Abdel-Aziz HR (2012) Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med 12(4):233–240
Mantawy EM, Bakly WM, Esmat A, Badr AM, Demerdash E (2014) Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 728:107–118
Kanoh S, Tanabe T, Rubin BK (2011) Dapsone inhibits IL-8 secretion from human bronchial epithelial cells stimulated with lipopolysaccharide and resolves airway inflammation in the ferret. Chest 140(4):980–990
Ryu S, Han HM, Song PI, Armstrong CA, Park Y (2015) Suppression of Propionibacterium acnes infection and the associated inflammatory response by the antimicrobial peptide P5 in mice. PLoS ONE ONE 10(7):e0132619
Abe M, Shimizu A, Yokoyama Y, Takeuchi Y, Ishikawa O (2008) A possible inhibitory action of diaminodiphenyl sulfone on tumour necrosis factor-α production from activated mononuclear cells on cutaneous lupus erythematosus. Clin Exp Dermatol Exp Dermatol 33(6):759–763
Zhou T, Zhao L, Zhan R, He Q, Tong Y, Tian X, Wang H, Zhang T, Fu Y, Sun Y (2014) Blood–brain barrier dysfunction in mice induced by lipopolysaccharide is attenuated by dapsone. Biochem Biophys Res Commun 453(3):419–424
Diaz-Ruiz A, Zavala C, Montes S, Ortiz-Plata A, Salgado-Ceballos H, Orozco-Suarez S, Nava-Ruiz C, Neri I, Severiano F, Ríos C (2008) Antioxidant, antiinflammatory and antiapoptotic effects of dapsone in a model of brain ischemia/reperfusion in rats. J Neurosci Res 86(15):3410–3419
Venditti P, Balestrieri M, De Leo T, Di Meo S (1998) Free radical involvement in doxorubicin-induced electrophysiological alterations in rat papillary muscle fibres. Cardiovasc Res 38(3):695–702
Liu G, Liu Y, Wang R, Hou T, Chen C, Zheng S, Dong Z (2016) Spironolactone attenuates doxorubicin-induced cardiotoxicity in rats. Cardiovasc Ther 34(4):216–224
Acknowledgements
We deeply appreciate Gilaranco Co. for preparing dapsone powder for our experimental investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The Ethical Committee of Tehran University of Medical Sciences approved all the in vivo work (IR.TUMS.MEDICINE.REC.1398.364) and they were in accordance with the National Institute of Health (NIH) Guidelines for the Care and Use of Laboratory Animals (HHS publication 85–23, 1985), legislation for the protection of animals used for scientific purposes (Directive 2010/63/EU).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheibani, M., Nezamoleslami, S., Faghir-Ghanesefat, H. et al. Cardioprotective effects of dapsone against doxorubicin-induced cardiotoxicity in rats. Cancer Chemother Pharmacol 85, 563–571 (2020). https://doi.org/10.1007/s00280-019-04019-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-04019-6