Abstract
Purpose
Weekly dose-dense paclitaxel with carboplatin every 3 weeks (dose-dense TC) provides good efficacy, and neoadjuvant chemotherapy is common for advanced-stage disease. However, it is unclear the efficacy and safety of dose-dense TC as neoadjuvant chemotherapy. Therefore, we evaluated neoadjuvant dose-dense TC chemotherapy for advanced-stage ovarian carcinoma.
Methods
We retrospectively reviewed cases of ovarian carcinoma that were not suited for primary debulking surgery (2003–2014). The patients received neoadjuvant dose-dense TC chemotherapy, followed by interval debulking surgery and adjuvant chemotherapy.
Results
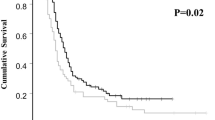
We identified 74 patients (mean age 60 years, range 39–85 years). The FIGO stages were IIIC (39/74, 52.7%) and IV (34/74, 45.9%). Fifty-six patients (75.6%) had a performance status of 0–1. The adverse events were grade 3/4 neutropenia (55.4%), anemia (44.6%), thrombocytopenia (21.6%), and peripheral neuropathy (8.1%); no treatment-related deaths were observed. Among the 66 patients who underwent debulking (89.2%), 55 patients (74.3%) achieved optimal debulking and 47 patients (63.5%) achieved complete resection. The median progression-free and overall survivals were 19.0 months (95% CI 16.2–23.7 months) and 55.1 months (95% CI 44.6 months to not estimable), respectively. A performance status of 2–3 was independently associated with poor prognosis (hazard ratio 3.84; p = 0.001).
Conclusions
Neoadjuvant dose-dense TC chemotherapy was effective (complete resection in >60% of cases) and tolerable for advanced-stage ovarian carcinoma.

Similar content being viewed by others
References
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E, Bielska-Lasota M, Engholm G, Nennecke A, Siesling S, Berrino F, Capocaccia R (2014) Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. Lancet Oncol 15(1):23–34. doi:10.1016/s1470-2045(13)70546-1
Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B, Crawford ED, Church TR, Andriole GL, Weissfeld JL, Fouad MN, Chia D, O’Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hartge P, Pinsky PF, Zhu CS, Izmirlian G, Kramer BS, Miller AB, Xu JL, Prorok PC, Gohagan JK, Berg CD (2011) Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA 305(22):2295–2303. doi:10.1001/jama.2011.766
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M (1996) Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 334(1):1–6. doi:10.1056/nejm199601043340101
du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, Bauknecht T, Richter B, Warm M, Schroder W, Olbricht S, Nitz U, Jackisch C, Emons G, Wagner U, Kuhn W, Pfisterer J (2003) A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 95(17):1320–1329
Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R (2003) Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 21(17):3194–3200. doi:10.1200/jco.2003.02.153
Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB, Kenter GG, Casado A, Mendiola C, Coens C, Verleye L, Stuart GC, Pecorelli S, Reed NS (2010) Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363(10):943–953. doi:10.1056/NEJMoa0908806
Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M, Dobbs S, Essapen S, Twigg J, Herod J, McCluggage G, Parmar M, Swart AM (2015) Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet (London, England) 386(9990):249–257. doi:10.1016/s0140-6736(14)62223-6
du Bois A, Herrstedt J, Hardy-Bessard AC, Muller HH, Harter P, Kristensen G, Joly F, Huober J, Avall-Lundqvist E, Weber B, Kurzeder C, Jelic S, Pujade-Lauraine E, Burges A, Pfisterer J, Gropp M, Staehle A, Wimberger P, Jackisch C, Sehouli J (2010) Phase III trial of carboplatin plus paclitaxel with or without gemcitabine in first-line treatment of epithelial ovarian cancer. J Clin Oncol 28(27):4162–4169. doi:10.1200/jco.2009.27.4696
Moebus V, Jackisch C, Lueck HJ, du Bois A, Thomssen C, Kurbacher C, Kuhn W, Nitz U, Schneeweiss A, Huober J, Harbeck N, von Minckwitz G, Runnebaum IB, Hinke A, Kreienberg R, Konecny GE, Untch M (2010) Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. J Clin Oncol 28(17):2874–2880. doi:10.1200/jco.2009.24.7643
Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP, Weiss AR (2012) Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol 30(33):4148–4154. doi:10.1200/jco.2011.41.5703
Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura E, Ochiai K, Noda K (2009) Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet (London, England) 374(9698):1331–1338. doi:10.1016/s0140-6736(09)61157-0
Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, Aoki D, Jobo T, Kodama S, Terauchi F, Sugiyama T, Ochiai K (2013) Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 14(10):1020–1026. doi:10.1016/s1470-2045(13)70363-2
Onda T, Kobayashi H, Nakanishi T, Hatae M, Iwasaka T, Konishi I, Shibata T, Fukuda H, Kamura T, Yoshikawa H (2009) Feasibility study of neoadjuvant chemotherapy followed by interval debulking surgery for stage III/IV ovarian, tubal, and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0206. Gynecol Oncol 113(1):57–62. doi:10.1016/j.ygyno.2008.12.027
Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ, Walker JL, DiSilvestro PA, Rubin SC, Martin LP, Davidson SA, Huh WK, O’Malley DM, Boente MP, Michael H, Monk BJ (2016) Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med 374(8):738–748. doi:10.1056/NEJMoa1505067
Hynninen J, Lavonius M, Oksa S, Grenman S, Carpen O, Auranen A (2013) Is perioperative visual estimation of intra-abdominal tumor spread reliable in ovarian cancer surgery after neoadjuvant chemotherapy? Gynecol Oncol 128(2):229–232. doi:10.1016/j.ygyno.2012.11.007
Acknowledgements
We thank all medical staffs who contributed to treatment of patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Ebata, T., Yunokawa, M., Bun, S. et al. Dose-dense paclitaxel plus carboplatin as neoadjuvant chemotherapy for advanced ovarian, fallopian tube, or primary peritoneal carcinomas. Cancer Chemother Pharmacol 78, 1283–1288 (2016). https://doi.org/10.1007/s00280-016-3187-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3187-3