Abstract
Purpose
Surgery is the only possible curative treatment for gastric cancer. However, the high recurrence rate makes gastric cancer difficult to cure by surgery alone. The present study was conducted to evaluate the clinical outcomes and toxicity of adjuvant treatment, including S-1/cisplatin chemotherapy followed by radiotherapy with concurrent S-1.
Methods
Patients with radically D2-resected adenocarcinoma of the stomach of stage IB–IV (M0) were eligible. Patients were treated with S-1 (40–60 mg depending on the patient’s body surface area) twice daily for 3 weeks and cisplatin (60 mg/m2) intravenously on day 1 every 5 weeks. Patients received CRT (45 Gy of radiation at 1.8 Gy/day, 5 days per week, for 5 weeks with the same dose of S-1 during radiation) followed by two additional cycles of S-1/cisplatin. The primary endpoint was the 3-year disease-free survival (DFS) rate; the secondary endpoints were the 3-year overall survival rate and toxicities.
Results
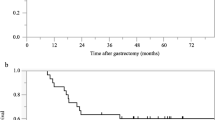
Until May 2012, 46 patients were enrolled, and 34 (73.9 %) completed the planned treatment. The median age was 53 years (range: 31–69 years), and the numbers of patients with stage IB, II, III and IV disease were 0, 17, 25 and 4, respectively. Main grade 3–4 toxicities were as follows: neutropenia (28.2 %), nausea (17.4 %), vomiting (8.7 %) and anorexia (15.2 %). At the time of analysis, after a median follow-up period of 56.5 months (3.03–74.0 months), 16 recurrence events and 15 deaths were reported. The estimated 3-year DFS and survival rates were 65.2 and 76.1 %, respectively. The most common site of recurrence was the peritoneum (n = 12).
Conclusions
The results of this phase II study show that intensified adjuvant treatment with S-1/cisplatin chemotherapy and S-1-based chemoradiotherapy was tolerable and effective in reducing disease recurrence. The addition of radiotherapy to chemotherapy may be effective in D2-resected gastric cancer. Although the data here are promising, a randomized trial is needed between patients treated with the current regimen and an appropriate comparator arm.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29. doi:10.3322/caac.21254
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345(10):725–730. doi:10.1056/NEJMoa010187
Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM, Stemmermann GN, Blanke CD, Macdonald JS (2012) Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 30(19):2327–2333. doi:10.1200/jco.2011.36.7136
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29(33):4387–4393. doi:10.1200/jco.2011.36.5908
Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ (2014) Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 15(12):1389–1396. doi:10.1016/s1470-2045(14)70473-5
Dai Q, Jiang L, Lin RJ, Wei KK, Gan LL, Deng CH, Guan QL (2015) Adjuvant chemoradiotherapy versus chemotherapy for gastric cancer: a meta-analysis of randomized controlled trials. J Surg Oncol 111(3):277–284. doi:10.1002/jso.23795
Bajetta E, Floriani I, Di Bartolomeo M, Labianca R, Falcone A, Di Costanzo F, Comella G, Amadori D, Pinto C, Carlomagno C, Nitti D, Daniele B, Mini E, Poli D, Santoro A, Mosconi S, Casaretti R, Boni C, Pinotti G, Bidoli P, Landi L, Rosati G, Ravaioli A, Cantore M, Di Fabio F, Aitini E, Marchet A (2014) Randomized trial on adjuvant treatment with FOLFIRI followed by docetaxel and cisplatin versus 5-fluorouracil and folinic acid for radically resected gastric cancer. Ann Oncol 25(7):1373–1378. doi:10.1093/annonc/mdu146
Min C, Bangalore S, Jhawar S, Guo Y, Nicholson J, Formenti SC, Leichman LP, Du KL (2014) Chemoradiation therapy versus chemotherapy alone for gastric cancer after R0 SURGICAL resection: a meta-analysis of randomized trials. Oncology 86(2):79–85. doi:10.1159/000354641
Huang YY, Yang Q, Zhou SW, Wei Y, Chen YX, Xie DR, Zhang B (2013) Postoperative chemoradiotherapy versus postoperative chemotherapy for completely resected gastric cancer with D2 Lymphadenectomy: a meta-analysis. PLoS One 8(7):e68939. doi:10.1371/journal.pone.0068939
Ajani JA, Buyse M, Lichinitser M, Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha R, Carrato A, Ferry D, Moiseyenko V (2013) Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer 49(17):3616–3624. doi:10.1016/j.ejca.2013.07.003
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, Forster G, McCloud PI (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20(4):666–673. doi:10.1093/annonc/mdn717
Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, Jung SH, Park CK, Kim KM, Kang WK (2012) Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 30(3):268–273. doi:10.1200/jco.2011.39.1953
Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, Sohn I, Jung SH, Choi MG, Lee JH, Bae JM, Kim S, Kim ST, Park JO, Park YS, Lim HY, Kang WK (2015) Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. doi:10.1200/jco.2014.58.3930
Lim DH, Kim DY, Kang MK, Kim YI, Kang WK, Park CK, Kim S, Noh JH, Joh JW, Choi SH, Sohn TS, Heo JS, Park CH, Park JO, Lee JE, Park YJ, Nam HR, Park W, Ahn YC, Huh SJ (2004) Patterns of failure in gastric carcinoma after D2 gastrectomy and chemoradiotherapy: a radiation oncologist’s view. Br J Cancer 91(1):11–17. doi:10.1038/sj.bjc.6601896
Costa WL Jr, Coimbra FJ, Fogaroli RC, Ribeiro HS, Diniz AL, Begnami MD, Mello CA, Fanelli MF, Silva MJ, Fregnani JH, Montagnini AL (2012) Adjuvant chemoradiotherapy after d2-lymphadenectomy for gastric cancer: the role of n-ratio in patient selection. results of a single cancer center. Radiat oncol 7:169. doi:10.1186/1748-717x-7-169
Zhu WG, Xua DF, Pu J, Zong CD, Li T, Tao GZ, Ji FZ, Zhou XL, Han JH, Wang CS, Yu CH, Yi JG, Su XL, Ding JX (2012) A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol 104(3):361–366. doi:10.1016/j.radonc.2012.08.024
Kim TH, Park SR, Ryu KW, Kim YW, Bae JM, Lee JH, Choi IJ, Kim YJ, Kim DY (2012) Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III–IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys 84(5):e585–e592. doi:10.1016/j.ijrobp.2012.07.2378
Kwon HC, Kim MC, Kim KH, Jang JS, Oh SY, Kim SH, Kwon KA, Lee S, Lee HS, Kim HJ (2010) Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac J Clin Oncol 6(4):278–285. doi:10.1111/j.1743-7563.2010.01331.x
Brooks GA, Enzinger PC, Fuchs CS (2012) Adjuvant therapy for gastric cancer: revisiting the past to clarify the future. J Clin Oncol 30(19):2297–2299. doi:10.1200/jco.2012.42.4069
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Funding
The study was sponsored by Jeil Pharmaceutical Co., Ltd.
Rights and permissions
About this article
Cite this article
Shim, HJ., Kim, KR., Hwang, JE. et al. A phase II study of adjuvant S-1/cisplatin chemotherapy followed by S-1-based chemoradiotherapy for D2-resected gastric cancer. Cancer Chemother Pharmacol 77, 605–612 (2016). https://doi.org/10.1007/s00280-016-2973-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-2973-2