Abstract
Purpose
Serum LDH, CEA, and CA19-9 levels are important tumor markers in pancreatic cancer. The purpose of this study was to evaluate the clinical significance of serum LDH, CEA, and CA19-9 levels in metastatic pancreatic cancer (MPC) receiving gemcitabine-based chemotherapy.
Materials and methods
In this retrospective study, we analyzed the outcome of 196 MPC patients who are treated with gemcitabine-based chemotherapy in our clinic.
Results
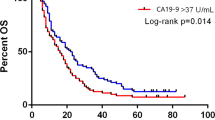
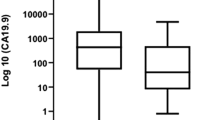
Positivity rates of serum LDH, CEA, and CA19-9 were 22, 40, and 83 %, respectively. Likewise, the rates of very high serum levels of tumor markers were correlated with these positivity rates (9 % for LDH, 30 % for CEA, and 55 % for CA19-9). The serum LDH levels were significantly higher in older patients (p = 0.05) and also in the patients with large tumors (p = 0.05), hepatic metastasis (p = 0.01), hypoalbuminemia (p = 0.01), and unresponsive to chemotherapy (p = 0.04). However, no correlation was found between both serum CEA and CA19-9 levels and possible prognostic factors (p > 0.05). The significant relationships were found between the serum levels of CEA and CA19-9 (r s = 0.24, p = 0.004), and serum LDH and CEA (r s = 0.193, p = 0.02). But, there was no correlation between serum LDH and CA19-9 levels (p = 0.39). One-year overall survival rate was 12.8 % (95 % CI 8–18). Increased serum levels of all the tumor markers significantly had adverse affect on survival (p = 0.001 for LDH, p = 0.002 for CEA, and p = 0.007 for CA19-9). However, no difference was observed in between high levels and very high levels of serum markers for all tumor markers (p > 0.05). Patients with normal serum levels of all three tumor markers had better outcome than others (p = 0.002) and those with normal serum LDH and CEA levels (whatever CA19-9) levels had associated with better survival compared with other possible alternatives (p < 0.001).
Conclusion
Serum levels of LDH, CEA, and CA19-9 had significant affect on survival in MPC patients.





Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Tas F, Aykan F, Alici S, Kaytan E, Aydiner A, Topuz E (2001) Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am J Clin Oncol 24:547–550
Haas M, Heinemann V, Kullmann F, Laubender RP, Klose C, Bruns CJ et al (2013) Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J Cancer Res Clin Oncol 139:681–689
Lee KJ, Yi SW, Chung MJ, Park SW, Song SY, Chung JB et al (2013) Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J 54:643–649
Stocken DD, Hassan AB, Altman DG, Billingham LJ, Bramhall SR, Johnson PJ et al (2008) Modeling prognostic factors in advanced pancreatic cancer. Br J Cancer 99:883–893
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tas, F., Karabulut, S., Ciftci, R. et al. Serum levels of LDH, CEA, and CA19-9 have prognostic roles on survival in patients with metastatic pancreatic cancer receiving gemcitabine-based chemotherapy. Cancer Chemother Pharmacol 73, 1163–1171 (2014). https://doi.org/10.1007/s00280-014-2450-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2450-8