Abstract
Purpose
Patients with gallbladder cancer or cholangiocarcinoma were treated with the combination of gemcitabine 1,000 mg/m2 IV over 100 min on days 1 and 8 and capecitabine 650 mg/m2 BID PO on days 1–14, administered every 21 days.
Methods
The primary objective of this study was to assess the response rate (confirmed complete and partial responses) of gemcitabine and capecitabine used in advanced/metastatic biliary neoplasms. Secondary objectives included overall survival and toxicities.
Results
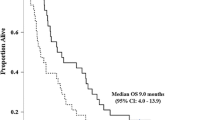
The study accrued 57 patients from September 2003 to April 2005. Three patients were ineligible, and two others received no treatment. Characteristics of analyzable patients: 35 (67%) cholangiocarcinoma, 17 (33%) gallbladder cancer; PS 0 (18 pts), 1 (26 pts), 2 (8 pts); 26 (50%) men; median age 58.8 years (29.5–85.6). Among 51 patients evaluated for toxicity, 6 experienced grade 4 toxicities. Among 52 patients, there were 7 confirmed partial responses for a confirmed response probability of 13% (95% CI: 6–26%). Six patients had an unconfirmed partial response for an overall response probability of 25% (95% CI: 14–39%). Twelve patients (23%) demonstrated stable disease. The 6-month overall survival was 55% (95% CI: 41–69%), and median survival was 7 months (95% CI: 5–8 months).
Conclusions
The combination of gemcitabine and capecitabine is a well-tolerated regimen with activity in patients with advanced gallbladder cancer and cholangiocarcinoma.


Similar content being viewed by others
References
American Cancer Society (2010). Cancer facts & figures. American Cancer Society, Atlanta
Serra I, Calvo A, Baez S et al (1996) Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer 78:1515–1517
Falkson G, MacIntyre JM, Moertel CG (1984) Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer 54:965–969
Takada T, Kato H, Matsushiro T et al (1996) Prospective randomized trial comparing 1/2 FAM (5-fluorouracil (5-FU) + adriamycin + mitomycin C) versus palliative therapy for the treatment of unresectable pancreatic and biliary tract carcinomas (the 2nd trial in non-resectable patients). Japanese Study Group of Surgical Adjuvant Therapy for Carcinomas of the Pancreas and Biliary Tract. Gan To Kagaku Ryoho 23:707–714
Kajanti M, Pyrhonen S (1994) Epirubicin-sequential methotrexate-5-fluorouracil-leucovorin treatment in advanced cancer of the extrahepatic biliary system. A phase II study. Am J Clin Oncol 17:223–226
Patt YZ, Jones DV Jr, Hoque A et al (1996) Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol 14:2311–2315
Sanz-Altamira PM, Ferrante K, Jenkins RL et al (1998) A phase II trial of 5-fluorouracil, leucovorin, and carboplatin in patients with unresectable biliary tree carcinoma. Cancer 82:2321–2325
Verderame F, Mandina P, Abruzzo F et al (2000) Biliary tract cancer: our experience with gemcitabine treatment. Anticancer Drugs 11:707–708
Castro MP (1998) Efficacy of gemcitabine in the treatment of patients with gallbladder carcinoma: a case report. Cancer 82:639–641
Eng C, Ramanathan RK, Wong MK et al (2004) A phase II trial of fixed dose rate gemcitabine in patients with advanced biliary tree carcinoma. Am J Clin Oncol 27:565–569
Valle J, Wasan H, Palmer DH et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96:896–902
Braakhuis BJ, Ruiz van Haperen VW, Boven E et al (1995) Schedule-dependent antitumor effect of gemcitabine in in vivo model system. Semin Oncol 22:42–46
Tempero M, Plunkett W, Ruiz Van Haperen V et al (2003) Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 21:3402–3408
Mandola MV, Stoehlmacher J, Zhang W et al (2004) A 6 bp polymorphism in the thymidylate synthase gene causes message instability and is associated with decreased intratumoral TS mRNA levels. Pharmacogenetics 14:319–327
Lurje G, Zhang W, Yang D et al (2008) Thymidylate synthase haplotype is associated with tumor recurrence in stage II and stage III colon cancer. Pharmacogenet Genomics 18:161–168
Riechelmann RP, Townsley CA, Chin SN et al (2007) Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 110:1307–1312
Cho JY, Paik YH, Chang YS et al (2005) Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer 104:2753–2758
Iyer RV, Gibbs J, Kuvshinoff B et al (2007) A phase II study of gemcitabine and capecitabine in advanced cholangiocarcinoma and carcinoma of the gallbladder: a single-institution prospective study. Ann Surg Oncol 14:3202–3209
Santini D, Vincenzi B, Vasile E et al (2009) Fixed dose rate (FDR) gemcitabine (G) and capecitabine (C) in patients with metastatic biliary tract cancer (BTC): final results of phase II trial. Abstract # e15510. Proceedings of the American Society of Clinical Oncology
Kim ST, Park JO, Lee J et al (2006) A phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 106:1339–1346
Julka PK, Puri T, Rath GK (2006) A phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary Pancreat Dis Int 5:110–114
André T, Reyes-Vidal JM, Fartoux L et al (2006) An international multicenter phase II trial of gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer. J Clin Oncol. ASCO annual meeting proceedings part I, vol 24, no. 18S (June 20 Supplement), p 4135
Acknowledgments
Support: This investigation was supported in part by the following Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute, Department of Health and Human Services: CA32102, CA38926, CA20319, CA58882, CA45808, CA58723, CA35176, CA76132, CA46441, CA63844, CA35178, CA58658, CA67575, CA45560, CA11083, CA46113, CA35128, CA58861, CA46368, CA35261, CA58416, CA42777, and CA35431 and supported in part by Roche.
Conflict of interest
Dr. Charles Blanke has consultant/advisory role for Roche Pharmaceuticals; Dr. El-Khoueiry has a consultant/advisory role and funding from Genentech.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iqbal, S., Rankin, C., Lenz, HJ. et al. A phase II trial of gemcitabine and capecitabine in patients with unresectable or metastatic gallbladder cancer or cholangiocarcinoma: Southwest Oncology Group study S0202. Cancer Chemother Pharmacol 68, 1595–1602 (2011). https://doi.org/10.1007/s00280-011-1657-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1657-1