Abstract
Background
Cytotoxicity of Vitamin K3 (VK3) is indicated to have the same mechanism with oxidative stress (H2O2). In the present study, we analyzed the differences and/or similarities in the cellular responses to oxidative stress and VK3 to clarify the mechanism of growth inhibition.
Methods
Cell viability was determined by a test method with 3-[4, 5-dimethyl-thiazol]-2, 5-dephenyl tetrazolium bromide (MTT). Expressions of cellular proteins were evaluated by Western blot analysis.
Results
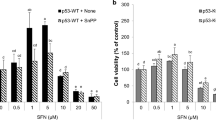
The IC50 was calculated to be 47.3 ± 4.1 μM for VK3 and 2.2 ± 1.2 μM for H2O2. By Western blot analysis, VK3 or H2O2 was shown to induce rapid phosphorylation of extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinases (JNKs). H2O2-induced phosphorylation of ERK and JNK was almost complete inhibited by more than 100-μM genistein. VK3-induced JNK phosphorylation was blocked by 100-μM genistein, but ERK phosphorylation was not inhibited completely even if 400-μM genistein was used. H2O2-induced inhibition of cell proliferation was completely blocked by 400-μM genistein, but the VK3 effect was reduced 72.8 ± 5.4% by the same concentration of genistein. H2O2-induced JNK phosphorylation and ERK phosphorylation were inhibited by staurosporine, protein kinase C (PKC) inhibitor. VK3-induced JNK phosphorylation was also blocked, but ERK phosphorylation was not affected. Staurosporine had no effect on VK3- or H2O2-induced growth inhibition. Treatment with a non-thiol antioxidant agent, catalase, completely abrogated H2O2-induced JNK and ERK phosphorylation, but a thiol antioxidant, l-cystein, had no effect on phosphorylation of them. The VK3-induced JNK phosphorylation was inhibited by catalase, but not l-cystein. But ERK phosphorylation was not inhibited by catalase and was abrogated completely by the thiol antioxidant. Catalase, but not l-cystein, blocked H2O2-induced growth inhibition, and l-cystein, but not catalase, blocked VK3-induced effects on cell proliferation completely.
Conclusion
VK3-induced ERK phosphorylation occurs by a different mechanism from oxidative stress, and it might have an important role to induce growth inhibition.





Similar content being viewed by others
References
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Cuzzocrea S, Thiemermann C, Salvemini D (2004) Potential therapeutic effect of antioxidant therapy in shock and inflammation. Curr Med Chem 11:1147–1162
Kovacic P, Jacintho JD (2001) Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem 8:773–796
Dong-Yun S, Yu-Ru D, Shan-Lin L, Ya-Dong Z, Lian W (2003) Redox stress regulates cell proliferation and apopstosis of human hepatoma though Akt protein phosphorylation. FEBS Lett 542:60–64
Sun Y, Oberley LW, Elwell JHm Sierra-Rivera E (1989) Antioxidant enzyme activities in normal and transformed mouse liver cells. Int J Cancer 44:1028–1033
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
Osada S, Imai H, Tomita H, et al (2006) Vascular endothelial growth factor protects hepatoma cells against oxidative stress-induced cell death. J Gastroenterol Hepatol 21:988–993
Dragin N, Smani M, Arnaud-Dabernat S, Dubost C, Moranvillier I, Costet P, Daniel JY, Peuchant E (2006) Acute oxidative stress is associated with cell proliferation in the mouse liver. FEBS Lett 580:3845–3852
Ma X, Du J, Nakashima I, Nagase F (2002) Menadione biphasically controls JNK-linked cell death in leukemia Jurkat T cells. Antioxid Redox Signal 4:371–378
Verrax J, Cadrobbi J, Delvaux M, Jamison JM, Gilloteaux J, Sumemrs JL (2003) The association of Vitamin C and K3 kills cancer cells mainly by autoschizis, a novel form of cell death. Basis for their potential use as coadjuvants in anticancer therapy. Eur J Med Chem 38:451–457
DeYulia GJ Jr, Carcamo JM, Borquez-Ojeda O, Shelton CC, Golde DW (2005) Hydrogen peroxide-generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc Natl Acad Sci USA 192:5044–5049
Buc Calderon P, Cadrobbi J, Marques C, Hong-Ngoc N, Jamison JM, Gilloteaux J, et al (2002) Potential therapeutic application of the association of vitamins C and K3 in cancer treatment. Curr Med Chem 9:2271–2285
Osada S, Kanematsu M, Imai H, et al (2005) Evaluation of extracellular signal regulated kinase expression ant its relation to treatment of hepatocellular carcinoma. J Am Coll Surg 201:405–411
Lee JM, Hanson JM, Chu WA, Johnson JA (2001) Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem 276:20011–20016
Huang HC, Nguyen T, Pickett CB (2002) Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem 277:42769–42774
Garrington TP, Johnson GL (1999) Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 11:211–218
Awazu M, Ishikura K, Hida M, Hoshiya M (1999) Mechanisms of mitogen-activated protein kinase activation in experimental diabetes. J Am Soc Nephrol 10:738–745
Han HJ, Heo JS, Lee YJ, Min JJ, Park KS (2006) High glucose-induced inhibition of 2-deoxyglucose uptake is mediated by c-AMP, protein kinase C, oxidative stress and mitogen-activated protein kinases in mouse embryonic cells. Clin Exper Pharm Phys 33:211–220
LeHoux JG, Lefebvre A (2006) Novel protein kinase C-epsilon inhibits human CYP11B2 gene expression through ERK 1/2 signaling pathway and JunB. J Mol Endoscin 36:51–64
Malarkey K, Belham CM, Paul A, et al (1995) The regulation of tyrosine kinase signaling pathways by growth factor and G-protein-coupled receptors. Biochem J 309:361–375
Ni CW, Wang DL, Lien SC, Cheng JJ, Chao YJ, Hsieh HJ (2003) Activation of PKC-epsilon and ERK 1/2 partcipates in shear-induced endothelial MCP-1 expression that is repressed by nitric oxide. J Cell Physiol 195:428–434
Kim KY, Choi KC, Auersperg N, Leung PC (2006) Mechanism of gonadotropin-releasing hormone (GnRH)-I and –II-induced cell growth inhibition in ovarian cancer cells: role of the GnRH-I receptor and protein kinase C pathway. Endocr Relat Cancer 13:211–220
Hecht D, Zick Y (1992) Selective inhibition of protein tyrosine phosphatase activities by H2O2 and vanadate in vitro. Biochem Biophys Res Commun 188:773–779
Nishikawa Y, Wang Z, Kerns J, Wilcox CS, Carr BI (1999) Inhibition of hepatoma cell growth in vitro by arylating and non-arylating K vitamin analogs. Significance of protein tyrosine phosphatase inhibition. J Biol Chem 274:34803–34810
Osada S, Saji S (2003) New approach to cancer therapy: the application of signal transduction to anti-cancer drug. Curr Med Chem 3:119–131
Kerns J, Naganathan S, Dowd P, Finn F, Carr BI (1995) Thioalkyl derivatives of vitamin K3 and vitamin K3 oxide inhibit growth of Hep 3B and Hep G2. Bioorg Chem 23:101–108
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greengerg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331
Osada S, Saji S, Osada K (2001) Critical role of extracellular signal-regulated kinase phosphorylation on menadione (vitamin K3) induced growth inhibition. Cancer 91:1156–1165
Osada S, Carr BI (2001) Mechanism of novel vitamin K analogs induced growth inhibition in human hepatoma cell line. J Hepatol 34:676–682
Zhu L, Yu X, Akatsuka Y, Cooper JA, Anasetti C (2001) Role of mitogen-activated protein kinases in activation-induced apoptosis of T cells. Immunology 92:26–35
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osada, S., Sakashita, F., Hosono, Y. et al. Extracellular signal-regulated kinase phosphorylation due to menadione-induced arylation mediates growth inhibition of pancreas cancer cells. Cancer Chemother Pharmacol 62, 315–320 (2008). https://doi.org/10.1007/s00280-007-0610-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0610-9