Abstract
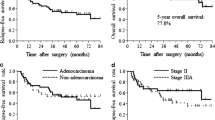
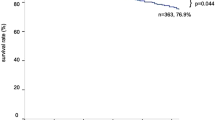
Two randomized phase III trials [uracil–tegafur (UFT) and bestatin trials] of adjuvant treatment for resected stage I non-small cell lung cancer (NSCLC) have recently been completed in Japan. The Japan Lung Cancer Research Group on Postsurgical Adjuvant Chemotherapy conducted a phase III trial in which 999 patients with completely resected stage I adenocarcinoma were assigned to receive either oral UFT [tegafur, 250 mg/(m2 day)] for 2 years or no treatment (January 1994–March 1997). At a median follow-up time of 73 months, the overall survival in the UFT group was significantly higher than that in the control group (P = 0.035). Grade 3 toxic effects occurred in 10 of the 482 patients (2%) who received UFT. Since 1985 when UFT became available in Japan, a total of 6 phase III trials comparing adjuvant chemotherapy using UFT with observation alone, including the above trial, have been conducted. A meta-analysis of these six trials reconfirmed that UFT had a beneficial effect in patients with completely resected stage I NSCLC. A phase III trial comparing UFT with platinum-based chemotherapy in patients with completely resected NSCLC with pathological stage IB through IIIA disease is also now under consideration. Bestatin is a potent aminopeptidase inhibitor that has both immunostimulant and antitumor activities. A prospective randomized, double blind, placebo-controlled trial in patients with completely resected stage I squamous cell carcinoma was conducted. A total of 402 patients were randomly assigned to receive either oral bestatin 30 mg/day or a placebo daily for 2 years (July 1992–March 1995). At the median follow-up time of 76 months, the overall survival in the bestatin group was significantly higher than that of the placebo control group (P = 0.033). The 5 year overall survival was 81% in the bestatin group and 74% in the placebo group. Few adverse events were observed in either group. These results, however, require further confirmation in other phase III trials.




Similar content being viewed by others
References
Ashmun RA, Look AT (1990) Metalloprotease activity of CD13/aminopeptidase N on the surface of human myeloid cells. Blood 75:462–469
Bhagwat SV, Lahdenranta J, Giordano R, Arap W, Pasqualini R, Shapiro LH (2001) CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 97:652–659
Burley S, David P, Lipcomb W (1991) Leucine aminopeptidase: bestatin inhibition and a model for enzyme-catalyzed peptide hydrolysis. Proc Natl Acad Sci USA 88:6919–6920
Endo C, Saito Y, Iwanami H, Tsushima T, Imai T, Kawamura M, Kondo T, Koike K, Handa M, Kanno R, Fujimura S (2003) A randomized trial of postoperative UFT therapy in p stage I, II non-small cell lung cancer: north-east Japan study group for lung cancer surgery. Lung Cancer 40:181–186
Ezawa K, Minato K, Dobashi K (1996) Induction of apoptosis by ubenimex (Bestatin) in human non-small-cell lung cancer cell lines. Biomed Pharmacother 50:283–289
Fujii S, Kitano S, Ikenaka K, Shirasaka T (1979) Effect of coadministration of uracil or cytosine on the anti-tumor activity of clinical doses of 1-(2-tetrahydrofuryl)-5-fluorouracil and level of 5-fluorouracil in rodents. Gann 70:209–214
Fujii H, Nakajima M, Aoyagi T, Tsuruo T (1996) Inhibition of tumor cell invasion and matrix degradation by aminopeptidase inhibitors. Biol Pharm Bull 19:6–10
Hamada C, Tanaka F, Ohta M, Fujimura S, Kodama K, Imaizumi M, Wada H (2005) Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol 23:4999–5006
Ho DH, Pazdur R, Covington W, Brown N, Huo YY, Lassere Y, Kuritani J (1998) Comparison of 5-fluorouracil pharmacokinetics in patients receiving continuous 5-fluorouracil infusion and oral uracil plus N1-(2′-tetrahydrofuryl)-5-fluorouracil. Clin Cancer Res 4:2085–2088
Ichinose Y, Genka K, Koike T, Kato H, Watanabe Y, Mori T, Iioka S, Sakuma A, Ohta M; NK421 Lung Cancer Surgery Group (2003) Randomized double-blind placebo-controlled trial of bestatin in patients with resected stage I squamous-cell lung carcinoma. J Natl Cancer Inst 95(8):605–610
Ikenaka K, Shirasaka T, Kitano S, Fujii S (1979) Effect of uracil on metabolism of 5-fluorouracil in vitro. Gann 70:353–359
Imaizumi M (2005) Postoperative adjuvant cisplatin, vindesine, plus uracil-tegafur chemotherapy increased survival of patients with completely resected p-stage I non-small cell lung cancer. Lung Cancer 49:85–94
Ino K, Bierman PJ, Varney ML, Heimann DG, Kuszynski CA, Walker SA, Talmadge JE (1996) Monocyte activation by an oral immunomodulator (bestatin) in lymphoma patients following autologous bone marrow transplantation. Cancer Immunol Immunother 43:206–212
Ishizuka M, Sato J, Sugiyama Y, Takeuchi T, Umezawa H (1980) Mitogenic effect of bestatin on lymphocytes. J Antibiot (Tokyo) 33:653–662
Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N, Ohta M; Japan Lung Cancer Research Group on Postsurgical Adjuvant Chemotherapy (2004) A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 350 (17):1713–1721
Leyhausen G, Schuster DK, Vaith P, Zahn RK, Umezawa H, Falke D, Muller WE (1983) Identification and properties of the cell membrane bound leucine aminopeptidase interacting with the potential immunostimulant and chemotherapeutic agent bestatin. Biochem Pharmacol 32:1051–1057
Look AT, Ashmun RA, Shapiro LH, Peiper SC (1989) Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest 83:1299–1307
Mountain CF (1986) A new international staging system for lung cancer. Chest 89:225S–233S
Muller WE, Schuster DK, Zahn RK, Maidhof A, Leyhausen G, Falke D, Koren R, Umezawa H (1982) Properties and specificity of binding sites for the immunomodulator bestatin on the surface of mammalian cells. Int J Immunopharmacol 4:393–400
Nakagawa M, Tanaka F, Tsubota N, Ohta M, Takao M, Wada H (2005) A randomized phase III trial of adjuvant chemotherapy with UFT for completely resected pathological stage I non-small-cell lung cancer: the West Japan Study Group for Lung Cancer Surgery (WJSG)–the 4th study. Ann Oncol 16:75–80
Okimoto N, Soejima R, Teramatsu T (1996) A randomized controlled postoperative adjuvant chemotherapy trial of CDDP + VDS + UFT and UFT alone in comparison with operation only for non-small cell lung carcinomas (second study). Jpn J Lung Cancer 36:863–871
Ota K, Kurita S, Yamada K, Masaoka T, Uzuka Y, Ogawa N (1986) Immunotherapy with bestatin for acute nonlymphocytic leukemia in adults. Cancer Immunol Immunother 23:5–10
Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E (2000) Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res 60:722–727
Razak K, Newland AC (1992) The significance of aminopeptidases and haematopoietic cell differentiation. Blood Rev 6:243–250
Riemann D, Kehlen A, Langner J (1999) CD13—not just a marker in leukemia typing. Immunol Today 20:83–88
Saiki I, Fujii H, Yoneda J, Abe F, Nakajima M, Tsuruo T, Azuma I (1993) Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int J Cancer 54:137–143
Shipp MA, Look AT (1993) Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood 82:1052–1070
Tada H, Yasumitsu T, Iuchi K, Taki T, Kodama K, Mori T (2002) Randomized study of adjuvant chemotherapy for completely resected non-small cell lung cancer. Proc Am Soc Clin Oncol 21:313a
Tartter PI, Burrows L, Kirschner P (1984) Perioperative blood transfusion adversely affects prognosis after resection of stage I (subset N0) non-oat cell lung cancer. J Thorac Cardiovasc Surg 88:659–662
Taylor A (1993) Aminopeptidases: structure and function. Faseb J 7:290–298
Umezawa H, Aoyagi T, Suda H, Hamada M, Takeuchi T (1976) Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot (Tokyo) 29:97–99
Urabe A, Mutoh Y, Mizoguchi H, Takaku F, Ogawa N (1993) Ubenimex in the treatment of acute nonlymphocytic leukemia in adults. Ann Hematol 67:63–66
Wada H, Hitomi S, Teramatsu T (1996) Adjuvant chemotherapy after complete resection in non-small-cell lung cancer. West Japan study group for lung cancer surgery. J Clin Oncol 14:1048–1054
Williams DE, Pairolero PC, Davis CS, Bernatz PE, Payne WS, Taylor WF, Uhlenhopp MA, Fontana RS (1981) Survival of patients surgically treated for stage I lung cancer. J Thorac Cardiovasc Surg 82:70–76
Yasumitsu T, Ohshima S, Nakano N, Kotake Y, Tominaga S (1990) Bestatin in resected lung cancer. A randomized clinical trial. Acta Oncol 29:827–831
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented at the 21st Bristol-Myers Squibb Nagoya International Cancer Treatment Symposium, “Lung Cancer: Novel Therapy against Malfunctioning Molecules”, 24–25 February 2006, Nagoya, Japan.
Rights and permissions
About this article
Cite this article
Ichinose, Y. A randomized phase III trial of adjuvant treatment for resected non-small cell lung cancer in Japan. Cancer Chemother Pharmacol 58 (Suppl 1), 43–48 (2006). https://doi.org/10.1007/s00280-006-0315-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0315-5