Abstract
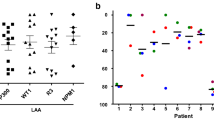
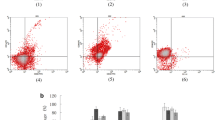
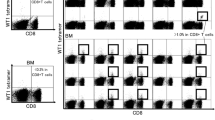
Recently, the focus is on new specific immunotherapies for AML such as cellular therapies employing dendritic cells (DCs) generated from AML blasts. AML-DCs express constitutionally leukemia-associated antigens (LAAs) present in AML blasts they are generated from. Here we investigated whether the generation of AML-DCs would alter the expression level of LAAs. Moreover, we evaluated the presence of HLA and costimulatory molecules on AML blasts versus AML-DCs. Quantitative real-time polymerase chain reaction (PCR) was performed for the following LAAs: preferentially expressed antigen in melanoma (PRAME), the receptor for hyaluronic acid mediated motility (RHAMM/CD168), Wilms’ tumor gene 1 (WT-1) and proteinase 3. The expression of HLA-ABC, HLA-DR, CD40, CD80, CD83 and CD86 was evaluated by flow cytometry. RHAMM protein expression was evaluated by immunocytochemistry, recognition of AML-DCs by PRAME epitope-specific T cells was evaluated in a chromium-release assay. Quantitative real-time PCR for AML-DCs versus AML blasts showed an alteration in mRNA expression of LAAs. An elevated PCR signal for PRAME was detected in 7/12 AML-DC preparations. 6/12 AML-DC preparations showed a significant upregulation of the PCR signal for RHAMM. A stronger WT-1 and proteinase-3 signal was observed in PCR for only 2/12 and 1/12 AML-DCs , respectively. All preparations showed a strong expression of at least one of the LAAs examined. As demonstrated by flow cytometry, AML-DCs strongly upregulated costimulatory molecules like CD40 and CD80 in comparison with AML blasts. AML-DCs tested positive for RHAMM protein. PRAME positive AML-DCs were recognized by specific T cells. AML-DCs might constitute a powerful tool in immunotherapy for AML. Real-time PCR allows a quick and quantitative assessment of immunologically relevant LAA expression with only 105 DCs and might be helpful for the decision whether the AML-DC vaccination strategy is favourable or not.








Similar content being viewed by others
References
Abetamann V, Kern HF, Elsasser HP (1996) Differential expression of the hyaluronan receptors CD44 and RHAMM in human pancreatic cancer cells. Clin Cancer Res 2:1607–1618
Banchereau J, Brière F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811
Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G (2001) Dendritic cells as vectors for therapy. Cell 106:271–274
Barth TF, Rinaldi N, Bruderlein S, Mechtersheimer G, Strater J, Altevogt P, Moller P (2002) Mesothelial cells in suspension expose an enriched integrin repertoire capable of capturing soluble fibronectin and laminin. Cell Commun Adhes 9:1–14
Bergmann L, Miething C, Maurer U, Brieger J, Karakas T, Weidmann E, Hoelzer D (1997) High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood 90:1217–1225
Bories D, Raynal MC, Solomon DH, Darzynkiewicz Z, Cayre YE (1989) Down-regulation of a serine protease, myeloblastin, causes gwoth arrest and differentiation of promyelocytic leukemia cells. Cell 59:959–968
Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH (1990) Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 60:509–520
Choudhury A, Gajewski JL, Liang JC, Popat U, Claxton DF, Kliche KO, Andreeff M, Champlin RE (1997) Use of leukemic dendritic cells for the generation of anti-leukemic cellular cytotoxicity against Philadelphia chromosome-positive chronic myelogenous leukemia. Blood 89:1133–1142
Choudhury A, Toubert A, Sutaria S, Charron D, Champlin RE, Claxton DF (1998) Human leukemia-derived dendritic cells: ex-vivo development of specific antileukemic cytotoxicity. Crit Rev Immunol 18:121–131
Choudhury BA, Liang JC, Thomas EK, Flores-Romo L, Xie QS, Agusala K, Sutaria S, Sinha I, Champlin RE, Claxton DF (1999) Dendritic cells derived in vitro from acute myelogenous leukemia cells stimulate autologous antileukemic T cell responses. Blood 93:780–786
Cignetti A, Bryant E, Allione B, Vitale A, Foa R, Cheever MA (1999) CD34(+) acute myeloid and lymphoid leukemic blasts can be induced to differentiate into dendritic cells. Blood 94:2048–2055
Crainie M, Belch AR, Mant MJ, Pilarski LM (1999) Overexpression of the receptor for hyaluronan- mediated motility (RHAMM) characterizes the malignant clone in multiple myeloma: identification of three distinct RHAMM variants. Blood 93:1684–1696
Dengler R, Munstermann U, al-Batran S, Hausner I, Faderl S, Nerl C, Emmerich B (1995) Immunocytochemical and flow cytometric detection of proteinase 3 (myeloblastin) in normal and leukaemic myeloid cells. Br J Haematol 89:250–257
Frohling S, Skelin S, Liebisch C, Scholl C, Schlenk RF, Dohner H, Dohner K (2002) Comparison of cytogenetic and molecular cytogenetic detection of chromosome abnormalities in 240 consecutive adult patients with acute myeloid leukemia. J Clin Oncol 20:2480–2485
Greiner J, Ringhoffer M, Taniguchi M, Hauser T, Schmitt A, Dohner H, Schmitt M (2003) Characterization of several leukemia-associated antigens inducing humoral immune responses in acute and chronic myeloid leukemia. Int J Cancer 106:224–231
Greiner J, Ringhoffer M, Taniguchi M, Schmitt A, Kirchner D, Krahn G, Heilmann V, Gschwend J, Bergmann L, Dohner H, Schmitt M (2002) Receptor for hyaluronan acid-mediated motility (RHAMM) is a new immunogenic leukemia- associated antigen in acute and chronic myeloid leukemia. Exp Hematol 30:1029–1035
Greiner J, Ringhoffer M, Szmaragowska A (eds) (2002) Quantitative measurement of the mRNA expression of the tumor-associated antigen PRAME by real-time RT-PCR using the lightcycler and SYBR green I technology. Rapid cycle real-time PCR methods and applications. Genetics and oncology. Springer, Berlin Heidelberg New York, pp 177–186
Hosen N, Sonoda Y, Oji Y, Kimura T, Minamiguchi H, Tamaki H, Kawakami M, Asada M, Kanato K, Motomura M, Murakami M, Fujioka T, Masuda T, Kim EH, Tsuboi A, Oka Y, Soma T, Ogawa H, Sugiyama H (2002) Very low frequencies of human normal CD34+ haematopoetic progenitor cells express the Wilms’ tumour gene WT1 at levels similar to those in leukaemia cells. Br J Haematol 116:409–420
Ikeda H, Lethe B, Lehmann F, van Baren N, Baurain JF, de Smet C, Chambost H, Vitale M, Moretta A, Boon T, Coulie PG (1997) Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 6:199–208
Kessler JH, Beekman NJ, Bres-Vloemans SA, Verdijk P, van Veelen PA, Kloosterman-Joosten AM, Vissers DC, ten Bosch GJ, Kester MG, Sijts A, Wouter Drijfhout J, Ossendorp F, Offringa R, Melief CJ (2001) Efficient identification of novel HLA-A*0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. J Exp Med 193:73–88
Li L, Schmitt A, Reinhardt P, Greiner J, Ringhoffer M, Vaida B, Bommer M, Vollmer M, Wiesneth M, Dohner H, Schmitt M (2003) Reconstitution of CD40 and CD80 in dendritic cells generated from blasts of patients with acute myeloid leukemia. Cancer Immun 3:8
Mailander V, Scheibenbogen C, Thiel E, Letsch A, Blau IW, Keilholz U (2004) Complete remission in a patient with recurrent acute myeloid leukemia induced by vaccination with WT1 peptide in the absence of hematological or renal toxicity. Leukemia 18:165–166
Matsushita M, Yamazaki R, Ikeda H, Kawakami Y (2003) Preferentially expressed antigen of melanoma (PRAME) in the development of diagnostic and therapeutic methods for hematological malignancies. Leuk Lymphoma 44:439–444
Maurer U, Weidmann E, Karakas T, Hoelzer D, Bergmann L (1997) Wilms tumor gene (wt1) mRNA is equally expressed in blast cells from acute myeloid leukemia and normal CD34+ progenitors. Blood 90:4230–4232
Molldrem J, Kant S, Lu S (2002) Peptide vaccination with PR1 elicits active T cell immunity that induces cytogenetic remission in acute myelogenous leukemia. Blood 98 abstract 8:92
Molldrem JJ, Lee PP, Kant S, Wieder E, Jiang W, Lu S, Wang C, Davis MM (2003) Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest 111:639–647
Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM (2000) Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med 6:1018–1023
Neumann E, Engelsberg A, Decker J, Storkel S, Jaeger E, Huber C, Seliger B (1998) Heterogeneous expression of the tumor-associated antigens RAGE-1, PRAME, and glycoprotein 75 in human renal cell carcinoma: candidates for T-cell-based immunotherapies? Cancer Res 58:4090–4095
Ohminami H, Yasukawa M, Fujita S (2000) HLA class I-restricted lysis of leukemia cells by a CD8+ cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood 95:286–293
Oka Y, Udaka K, Tsuboi A, Elisseeva OA, Ogawa H, Aozasa K, Kishimoto T, Sugiyama H (2000) Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol 164:1873–1880
Pilarski LM, Masellis-Smith A, Belch AR, Yang B, Savani RC, Turley EA (1994) RHAMM, a receptor for hyaluronan-mediated motility, on normal human lymphocytes, thymocytes and malignant B cells: a mediator in B cell malignancy? Leuk Lymphoma 14:363–374
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor. J Exp Med 179:1109–1118
Scheibenbogen C, Letsch A, Thiel E, Schmittel A, Mailaender V, Baerwolf S, Nagorsen D, Keilholz U (2002) CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood 100:2132–2137
Schmitt M, Ikeda H, Nagata Y, Gu X, Wang L, Kuribayashi K, Shiku H (1997) Involvement of T-cell subsets and natural killer (NK) cells in the growth suppression of murine fibrosarcoma cells transfected with interleukin-12 (IL-12) genes. Int J Cancer 72:505–511
Sherman L, Sleeman J, Herrlich P, Ponta H (1994) Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol 6:726–733
Stockerl-Goldstein KG, Blume KG (eds) (1999) Allogeneic hematopoietic cell transplantation for adult patients with acute myeloid leukemia. In: Thomas ED, Blume KG, Forman SJ (eds) Hematopoietic cell transplantation, 2nd edn, pp 823–834
Teder P, Bergh J, Heldin P (1995) Functional hyaluronan receptors are expressed on a squamous cell lung carcinoma cell line but not on other lung carcinoma cell lines. Cancer Res 55:3908–3914
van Baren N, Chambost H, Ferrant A, Michaux L, Ikeda H, Millard I, Olive D, Boon T, Coulie PG (1998) PRAME, a gene encoding an antigen recognized on a human melanoma by cytolytic T cells, is expressed in acute leukaemia cells. Br J Haematol 102:1376–1379
Vollmer M, Li L, Schmitt A, Greiner J, Reinhardt P, Ringhoffer M, Wiesneth M, Dohner H, Schmitt M (2003) Expression of human leucocyte antigens and and costimulatory antigens on blasts of patients with acute myeloid leukaemia. Br J Haematol 120:1000–1008
Wang C, Entwistle J, Hou G, Li Q, Turley EA (1996) The characterization of a human RHAMM cDNA: conservation of the hyaluronan-binding domains. Gene 174:299–306
Yang B, Zhang L, Turley EA (1993) Identification of two hyaluronan-binding domains in the hyaluronan receptor RHAMM. J Biol Chem 268:8617–8623
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, L., Reinhardt, P., Schmitt, A. et al. Dendritic cells generated from acute myeloid leukemia (AML) blasts maintain the expression of immunogenic leukemia associated antigens. Cancer Immunol Immunother 54, 685–693 (2005). https://doi.org/10.1007/s00262-004-0631-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-004-0631-8