Abstract
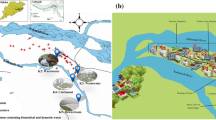
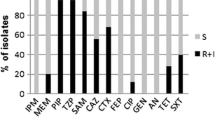
Acinetobacter spp. are ubiquitous bacteria in the environment. Acinetobacter spp. isolated from a municipal drinking water treatment plant and from connected tap water were identified to the species level on the basis of rpoB gene partial sequence analysis. Intraspecies variation was assessed based on the analysis of partial sequences of housekeeping genes (rpoB, gyrB, and recA). Antibiotic resistance was characterized using the disk diffusion method and isolates were classified as wild or non-wild type (non-WT), according to the observed phenotype. The strains of Acinetobacter spp. were related to 11 different validly published species, although three groups of isolates, presenting low rpoB sequence similarities with previously described species, may represent new species. Most of the isolates were related to the species A. johnsonii and A. lwoffii. These two groups, as well as others related to the species A. parvus and A. tjernbergiae, were detected in the water treatment plant and in tap water. Other strains, related to the species A. pittii and A. beijerinckii, were isolated only from tap water. Most of the isolates (80 %) demonstrated wild type (WT) to all of the 12 antibiotics tested. Non-WT for tetracycline, meropenem, and ceftazidime, among others, were observed in water treatment plant or in tap water samples. Although, in general, this study suggests a low prevalence of acquired antibiotic resistance in water Acinetobacter spp., the potential of some species to acquire and disseminate resistance via drinking water is suggested.



Similar content being viewed by others
References
Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, Rodriguez-Valera F (2005) Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 43:4382–4390
Bergogne-Berezin E, Towner KJ (1996) Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev 9:148–165
Bhargava N, Sharma P, Capalash N (2010) Quorum sensing in Acinetobacter: an emerging pathogen. Crit Rev Microbiol 36:349–360
Dhakephalkar PK, Chopade BA (1994) High levels of multiple metal resistance and its correlation to antibiotic resistance in environmental isolates of Acinetobacter. Biometals 7:67–74
Dimopoulou ID, Kartali SI, Kartalis GN, Manolas KI, Simopoulos KE, Vargemezis BA, Theodoropoulou-Rodiou G, Bowler IC, Crook DW (2003) Relationship between nosocomial Acinetobacter species occurring in two geographical areas (Greece and the UK). J Hosp Infect 54:207–211
Eichler S, Christen R, Holtje C, Westphal P, Botel J, Brettar I, Mehling A, Hofle MG (2006) Composition and dynamics of bacterial communities of a drinking water supply system as assessed by RNA- and DNA-based 16S rRNA gene fingerprinting. Appl Environ Microbiol 72:1858–1872
Figueira V, Vaz-Moreira I, Silva M, Manaia CM (2011) Diversity and antibiotic resistance of Aeromonas spp. In drinking and waste water treatment plants. Water Res 45:5599–5611
Fishbain J, Peleg AY (2010) Treatment of Acinetobacter infections. Clin Infect Dis 51:79–84
Gonzalez CJ, Santos JA, Garcia-Lopez ML, Otero A (2000) Psychrobacters and related bacteria in freshwater fish. J Food Prot 63:315–321
Gundi VA, Dijkshoorn L, Burignat S, Raoult D, La Scola B (2009) Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiol 155:2333–2341
Hantsis-Zacharov E, Halpern M (2007) Culturable psychrotrophic bacterial communities in raw milk and their proteolytic and lipolytic traits. Appl Environ Microbiol 73:7162–7168
Henwood CJ, Gatward T, Warner M, James D, Stockdale MW, Spence RP, Towner KJ, Livermore DM, Woodford N (2002) Antibiotic resistance among clinical isolates of Acinetobacter in the UK, and in vitro evaluation of tigecycline (gar-936). J Antimicrob Chemother 49:479–487
Hoefel D, Monis PT, Grooby WL, Andrews S, Saint CP (2005) Profiling bacterial survival through a water treatment process and subsequent distribution system. J Appl Microbiol 99:175–186
Hong PY, Hwang C, Ling F, Andersen GL, LeChevallier MW, Liu WT (2010) Pyrosequencing analysis of bacterial biofilm communities in water meters of a drinking water distribution system. Appl Environ Microbiol 76:5631–5635
Idzenga D, Schouten MA, van Zanten AR (2006) Outbreak of Acinetobacter genomic species 3 in a Dutch intensive care unit. J Hosp Infect 63:485–487
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism, edn. Academic Press, New York, pp 21–132
Juni E (2005) Proteobacteria, part b, the gammaproteobacteria. In: Brenner DJ, Krieg NR, Stanley JT (eds) Bergey's manual of systematic bacteriology, 2nd edn. Springer, New York
Kappstein I, Grundmann H, Hauer T, Niemeyer C (2000) Aerators as a reservoir of Acinetobacter junii: an outbreak of bacteraemia in paediatric oncology patients. J Hosp Infect 44:27–30
Kim MH, Hao OJ, Wang NS (1997) Acinetobacter isolates from different activated sludge processes: characteristics and neural network identification. FEMS Microbiol Ecol 23:217–227
Kormas KA, Neofitou C, Pachiadaki M, Koufostathi E (2010) Changes of the bacterial assemblages throughout an urban drinking water distribution system. Environ Monit Assess 165:27–38
Krawczyk B, Lewandowski K, Kur J (2002) Comparative studies of the Acinetobacter genus and the species identification method based on the recA sequences. Mol Cell Probes 16:1–11
Kuo HY, Yang CM, Lin MF, Cheng WL, Tien N, Liou ML (2010) Distribution of blaOXA-carrying imipenem-resistant Acinetobacter spp. in 3 hospitals in Taiwan. Diagn Microbiol Infect Dis 66:195–199
La Scola B, Gundi VA, Khamis A, Raoult D (2006) Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol 44:827–832
Clinical and Laboratory Standards Institute (2007) Performance standards for antimicrobial susceptibility testing m100-s17. 27: 2
Leclerc H, Moreau A (2002) Microbiological safety of natural mineral water. FEMS Microbiol Rev 26:207–222
Li D, Li Z, Yu J, Cao N, Liu R, Yang M (2010) Characterization of bacterial community structure in a drinking water distribution system during an occurrence of red water. Appl Environ Microbiol 76:7171–7180
Loret JF, Greub G (2010) Free-living amoebae: biological by-passes in water treatment. Int J Hyg Environ Health 213:167–175
Marshall C, Richards M, Black J, Sinickas V, Dendle C, Korman T, Spelman D (2007) A longitudinal study of Acinetobacter in three Australian hospitals. J Hosp Infect 67:245–252
McKeon DM, Calabrese JP, Bissonnette GK (1995) Antibiotic resistant gram-negative bacteria in rural groundwater supplies. Water Res 29:1902–1908
Medina J, Formento C, Pontet J, Curbelo A, Bazet C, Gerez J, Larranaga E (2007) Prospective study of risk factors for ventilator-associated pneumonia caused by Acinetobacter species. J Crit Care 22:18–26
Montealegre MC, Maya JJ, Correa A, Espinal P, Mojica MF, Ruiz SJ, Rosso F, Vila J, Quinn JP, Villegas MV (2012) First identification of OXA-72 carbapenemase from Acinetobacter pittii in Colombia. Antimicrob Agents Chemother. doi:10.1128/AAC.05628-11
Norton CD, LeChevallier MW (2000) A pilot study of bacteriological population changes through potable water treatment and distribution. Appl Environ Microbiol 66:268–276
Nowak A, Kur J (1995) Genomic species typing of acinetobacters by polymerase chain reaction amplification of the recA gene. FEMS Microbiol Lett 130:327–332
Pavlov D, de Wet CM, Grabow WO, Ehlers MM (2004) Potentially pathogenic features of heterotrophic plate count bacteria isolated from treated and untreated drinking water. Int J Food Microbiol 92:275–287
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Poirel L, Mansour W, Bouallegue O, Nordmann P (2008) Carbapenem-resistant Acinetobacter baumannii isolates from Tunisia producing the OXA-58-like carbapenem-hydrolyzing oxacillinase OXA-97. Antimicrob Agents Chemother 52:1613–1617
Poitelon JB, Joyeux M, Welte B, Duguet JP, Prestel E, Lespinet O, DuBow MS (2009) Assessment of phylogenetic diversity of bacterial microflora in drinking water using serial analysis of ribosomal sequence tags. Water Res 43:4197–4206
Quinteira S, Grosso F, Ramos H, Peixe L (2007) Molecular epidemiology of imipenem-resistant Acinetobacter haemolyticus and Acinetobacter baumannii isolates carrying plasmid-mediated OXA-40 from a Portuguese hospital. Antimicrob Agents Chemother 51:3465–3466
Regalado NG, Martin G, Antony SJ (2009) Acinetobacter lwoffii: bacteremia associated with acute gastroenteritis. Travel Med Infect Dis 7:316–317
Revetta RP, Pemberton A, Lamendella R, Iker B, Santo Domingo JW (2010) Identification of bacterial populations in drinking water using 16S rRNA-based sequence analyses. Water Res 44:1353–1360
Seifert H, Dijkshoorn L, Gerner-Smidt P, Pelzer N, Tjernberg I, Vaneechoutte M (1997) Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J Clin Microbiol 35:2819–2825
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Simões LC, Simões M, Vieira MJ (2010) Influence of the diversity of bacterial isolates from drinking water on resistance of biofilms to disinfection. Appl Environ Microbiol 76:6673–6679
Spence RP, Towner KJ, Henwood CJ, James D, Woodford N, Livermore DM (2002) Population structure and antibiotic resistance of Acinetobacter DNA group 2 and 13TU isolates from hospitals in the UK. J Med Microbiol 51:1107–1112
Thangaraj K, Kapley A, Purohit HJ (2008) Characterization of diverse Acinetobacter isolates for utilization of multiple aromatic compounds. Bioresour Technol 99:2488–2494
Thomas V, Herrera-Rimann K, Blanc DS, Greub G (2006) Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl Environ Microbiol 72:2428–2438
Thomas V, Loret JF, Jousset M, Greub G (2008) Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ Microbiol 10:2728–2745
Thomas V, McDonnell G, Denyer SP, Maillard JY (2010) Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol Rev 34:231–259
Tognim MC, Andrade SS, Silbert S, Gales AC, Jones RN, Sader HS (2004) Resistance trends of Acinetobacter spp. In Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the sentry antimicrobial surveillance program. Int J Infect Dis 8:284–291
Towner KJ (2009) Acinetobacter: An old friend, but a new enemy. J Hosp Infect 73:355–363
Van Looveren M, Goossens H, Steering Group ARPAC (2004) Antimicrobial resistance of Acinetobacter spp. in Europe. Clin Microbiol Infect 10:684–704
Vanbroekhoven K, Ryngaert A, Wattiau P, Mot R, Springael D (2004) Acinetobacter diversity in environmental samples assessed by 16S rRNA gene PCR-DGGE fingerprinting. FEMS Microbiol Ecol 50:37–50
Vaz-Moreira I, Egas C, Nunes OC, Manaia CM (2011a) Culture-dependent and culture-independent diversity surveys target different bacteria: a case study in a freshwater sample. Antonie Van Leeuwenhoek 100:245–257
Vaz-Moreira I, Novo A, Hantsis-Zacharov E, Lopes AR, Gomila M, Nunes OC, Manaia CM, Halpern M (2011b) Acinetobacter rudis sp. nov. isolated from raw milk and raw wastewater. Int J Syst Evol Microbiol 61:2837–2843
Vaz-Moreira I, Nunes OC, Manaia CM (2011c) Diversity and antibiotic resistance patterns of Sphingomonadaceae isolates from drinking water. Appl Environ Microbiol 77:5697–5706
Vaz-Moreira I, Nunes OC, Manaia CM (2012) Diversity and antibiotic resistance in Pseudomonas spp. from drinking water. Sci Total Environ 426:366–374
Villarreal JV, Schwartz T, Obst U (2010) Culture-independent techniques applied to food industry water surveillance—a case study. Int J Food Microbiol 141:S147–S155
Visca P, Seifert H, Towner KJ (2011) Acinetobacter infection—an emerging threat to human health. IUBMB Life 63:1048–1054
World Health Organisation (2008) Guidelines for drinking water. World Health Organization, Geneva
Xi C, Zhang Y, Marrs CF, Ye W, Simon C, Foxman B, Nriagu J (2009) Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl Environ Microbiol 75:5714–5718
Xu X, Kong F, Cheng X, Yan B, Du X, Gai J, Ai H, Shi L, Iredell J (2008) Integron gene cassettes in Acinetobacter spp. strains from south china. Int J Antimicrob Agents 32:441–445
Yamamoto S, Bouvet PJ, Harayama S (1999) Phylogenetic structures of the genus Acinetobacter based on gyrB sequences: comparison with the grouping by DNA-DNA hybridization. Int J Syst Bacteriol 49(Pt 1):87–95
Zhang Y, Marrs CF, Simon C, Xi C (2009) Wastewater treatment contributes to selective increase of antibiotic resistance among Acinetobacter spp. Sci Total Environ 407:3702–3706
Acknowledgments
The authors gratefully acknowledge the workers from the water treatment plant for their kind collaboration on sample collections and the people that kindly allowed the sampling of water from their taps.
This study was financed by Fundação para a Ciência e a Tecnologia (projects PTDC/AMB/70825/2006, PTDC/AAC-AMB/113840/2009 and IVM grant SFRH/BD/27978/2006). CNR was supported by ERASMUS placement agreement. LS-S and ERBM were supported, in part, by funding of the ALF-medel för forskning Västra Götaland Region project ALFGBG-11574 and the FoU-Västra Götaland Region project VGFOUREG-157801.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narciso-da-Rocha, C., Vaz-Moreira, I., Svensson-Stadler, L. et al. Diversity and antibiotic resistance of Acinetobacter spp. in water from the source to the tap. Appl Microbiol Biotechnol 97, 329–340 (2013). https://doi.org/10.1007/s00253-012-4190-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4190-1