Abstract
Because most medications for pediatric pulmonary hypertension (PH) are used off label and based on adult trials, little information is available on pediatric-specific adverse events (AEs). Although drug manufacturers are required to submit postmarket AE reports to the Food and Drug Administration (FDA), this information is rarely transmitted to practitioners. In the setting of a recent FDA warning for sildenafil, the authors sought to give a better description of the AEs associated with current therapies in pediatric PH. In January 2010, a written request was made to the Food and Drug Administration for AE records of commonly used PH medications. Reports were screened for pediatric patients, analyzed in terms of AEs, and compared with the medical literature. Arbitrarily, AEs that could be attributed to concomitant medications were not attributed to the PH medication in question. Adverse events occurring in more than 5 % of events for each drug were assumed to be associated with the targeted PH medication. Between November 1997 and December 2009, 588 pediatric AE reports (death in 257 cases) were reported for the three most commonly used therapies: bosentan, epoprostenol, and sildenafil. Many of the AEs were similar to those reported previously. However, 27 AEs not previously reported in the literature (e.g., pulmonary hemorrhage, hemoptysis, and pneumonia) were found. The FDA postmarket records for PH medications in pediatric patients show a significant number of AEs. The discovery of AEs not previously reported will better inform those caring for these complex and critically ill children, and the large number of deaths suggest they may be underreported in current literature.

Similar content being viewed by others
References
Abman S (2010) Pulmonary hypertension in children: a historical overview. Pediatr Crit Care Med 11(2 Suppl):S4–S9
Adverse Event Reporting System (AERS) (2006) Background report definitions, and caveats. Department of Health and Human Services
Barst RJ et al (2006) Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J 28:1195–1203
Barst RJ, Layton G, Konourina I, Richardson H, Beghetti M, Ivy DD (2012) STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive patients with pediatric pulmonary arterial hypertension. Eur Heart J 33(Suppl 1):979
Barst RJ et al (2012) A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 125:324–334
Cassens BJ (2010) Warning letters. Actelion Pharmaceuticals U.S., Inc. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm227632.htm. Accessed 1 Jun 2011
FDA (2012) FDA drug safety communication: FDA recommends against use of Revatio (sildenafil) in children with pulmonary hypertension. http://www.fda.gov/Drugs/DrugSafety/ucm317123.htm. Accessed 10 Jan 2013
Gilead Sciences (2007) I. U.S. Food and Drug Administration approves Gilead’s Letairis™ (ambrisentan) 5 mg and 10 mg tablets for the once-daily treatment of pulmonary arterial hypertension (WHO group 1) in patients with WHO functional class II or III symptoms. http://www.gilead.com/wt/sec/pr_1016053. Accessed 1 Jun 2011
McLaughlin VV et al (1998) Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. New Engl J Med 338:273–277
United Therapeutics (2009) FDA approves adcirca (Tadalafil) tablets for the treatment of pulmonary arterial hypertension. http://ir.unither.com/releasedetail.cfm?. Accessed 1 Jun 2011
Acknowledgments
We thank Raymond R. Balise, PhD, for his assistance in the statistical analysis.
Disclosure
The University of Colorado receives fees for Dr. Ivy to be a consultant for Actelion, Gilead, Pfizer and United Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
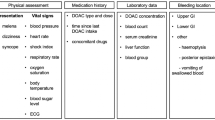
Example of MEDWATCH form


Appendix 2
Example of cross-referenced concomitant medications

In this example, dyspepsia, headache, and insomnia have been associated with sildenafil, anxiety with epoprostenol, and chest pain with bosentan. The remaining events of hemoptysis and muscle tightness are thus assumed to be associated with the targeted pulmonary hypertension medication
Rights and permissions
About this article
Cite this article
Maxey, D.M., Ivy, D.D., Ogawa, M.T. et al. Food and Drug Administration (FDA) Postmarket Reported Side Effects and Adverse Events Associated with Pulmonary Hypertension Therapy in Pediatric Patients. Pediatr Cardiol 34, 1628–1636 (2013). https://doi.org/10.1007/s00246-013-0688-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-013-0688-2