Abstract
Purpose
The purpose of the present study was to determine whether apparent brain temperature imaging using multi-voxel proton magnetic resonance (MR) spectroscopy correlates with cerebral blood flow (CBF) and metabolism imaging in the deep white matter of patients with unilateral chronic major cerebral artery steno-occlusive disease.
Methods
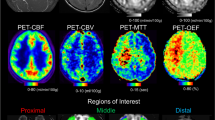
Apparent brain temperature and CBF and metabolism imaging were measured using proton MR spectroscopy and 15O-positron emission tomography (PET), respectively, in 35 patients. A set of regions of interest (ROIs) of 5 × 5 voxels was placed on an MR image so that the voxel row at each edge was located in the deep white matter of the centrum semiovale in each cerebral hemisphere. PET images were co-registered with MR images with these ROIs and were re-sliced automatically using image analysis software.
Results
In 175 voxel pairs located in the deep white matter, the brain temperature difference (affected hemisphere − contralateral hemisphere: ΔBT) was correlated with cerebral blood volume (CBV) (r = 0.570) and oxygen extraction fraction (OEF) ratios (affected hemisphere/contralateral hemisphere) (r = 0.641). We excluded voxels that contained ischemic lesions or cerebrospinal fluid and calculated the mean values of voxel pairs in each patient. The mean ΔBT was correlated with the mean CBF (r = − 0.376), mean CBV (r = 0.702), and mean OEF ratio (r = 0.774).
Conclusions
Apparent brain temperature imaging using multi-voxel proton MR spectroscopy was correlated with CBF and metabolism imaging in the deep white matter of patients with unilateral major cerebral artery steno-occlusive disease.



Similar content being viewed by others
References
Gibbs JM, Wise RJ, Leenders KL, Jones T (1984) Evaluation of cerebral perfusion reserve in patients with carotid-artery occlusion. Lancet 1:310–314
Powers WJ, Raichle ME (1985) Positron emission tomography and its application to the study of cerebrovascular disease in man. Stroke 16:361–376
Baron JC, Bousser MG, Rey A, Guillard A, Comar D et al (1981) Reversal of focal ‘misery-perfusion syndrome’ by extra-intracranial arterial bypass in hemodynamic cerebral ischemia: a case study with 15O positron emission tomography. Stroke 12:454–459
Yamauchi H, Higashi T, Kagawa S, Nishii R, Kudo T et al (2012) Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease? Brain 135:2515–2526
Kluytmans M, van der Grond J, Folkers PJ, Mali WP, Viergever MA (1998) Differentiation of gray matter and white matter perfusion in patients with unilateral internal carotid artery occlusion. J Magn Reson Imaging 8:767–774
Momjian-Mayor I, Baron JC (2005) The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Stroke 36:567–577
Moody DM, Bell MA, Challa VR (1990) Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol 11:431–439
Cady EB, D’Souza PC, Penrice J, Lorek A (1995) The estimation of local brain temperature by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 33:862–867
Corbett RJ, Laptook A, Weatherall P (1997) Noninvasive measurements of human brain temperature using volume-localized proton magnetic resonance spectroscopy. J Cereb Blood Flow Metab 17:363–369
Jayasundar R, Singh VP (2002) In vivo temperature measurements in brain tumors using proton MR spectroscopy. Neurol India 50:436–439
Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K et al (2006) Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Ann Neurol 60:438–446
Nybo L, Secher NH, Nielsen B (2002) Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol 545:697–704
Ishigaki D, Ogasawara K, Yoshioka Y, Chida K, Sasaki M et al (2009) Brain temperature measured using proton MR spectroscopy detects cerebral hemodynamic impairment in patients with unilateral chronic major cerebral artery steno-occlusive disease: comparison with positron emission tomography. Stroke 40:3012–3016
Frackowiak RS, Lenzi GL, Jones T, Heather JD (1980) Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr 4:727–736
Lammertsma AA, Jones T (1983) Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain: 1. Description of the method. J Cereb Blood Flow Metab 3:416–424
Yoshioka Y, Oikawa H, Ehara S, Inoue T, Ogawa A et al (2005) Noninvasive measurement of temperature and fractional dissociation of imidazole in human lower leg muscles using 1H-nuclear magnetic resonance spectroscopy. J Appl Physiol 98:282–287
Yoshioka Y, Shimada R, Oikawa H, Ehara S, Inoue T et al (2003) Evaluation of noninvasive measurement of human brain temperature using 1H magnetic resonance spectroscopy at 3T. J Iwate Med Assoc 55:377–384
Aoi MC, Hu K, Lo MT, Selim M, Olufsen MS et al (2012) Impaired cerebral autoregulation is associated with brain atrophy and worse functional status in chronic ischemic stroke. PLoS One 7:e46794
Segawa H, Wakai S, Tamura A, Yoshimasu N, Nakamura O et al (1983) Computed tomographic measurement of local cerebral blood flow by xenon enhancement. Stroke 14:356–362
Sato Y, Ogasawara K, Kuroda H, Suzuki T, Chida K et al (2011) Preoperative central benzodiazepine receptor binding potential and cerebral blood flow images on SPECT predict development of new cerebral ischemic events and cerebral hyperperfusion after carotid endarterectomy. J Nucl Med 52:1400–1407
Kawai N, Hatakeyama T, Okauchi M, Kawanishi M, Shindo A et al (2014) Cerebral blood flow and oxygen metabolism measurements using positron emission tomography on the first day after carotid artery stenting. J Stroke Cerebrovasc Dis 23:e55–e64
Derdeyn CP, Khosla A, Videen TO, Fritsch SM, Carpenter DL et al (2001) Severe hemodynamic impairment and border zone--region infarction. Radiology 220:195–201
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded in part by a Grant-in-Aid for Strategic Medical Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (S1491001) and Scientific Research from the Japan Society for the Promotion of Science (JP15K10313).
Conflict of interest
KO has received research funds from Nihon Medi-Physics Co., Ltd. and Bristol-Myers Squibb.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nanba, T., Nishimoto, H., Yoshioka, Y. et al. Apparent brain temperature imaging with multi-voxel proton magnetic resonance spectroscopy compared with cerebral blood flow and metabolism imaging on positron emission tomography in patients with unilateral chronic major cerebral artery steno-occlusive disease. Neuroradiology 59, 923–935 (2017). https://doi.org/10.1007/s00234-017-1890-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1890-3