Abstract
Purpose
The aims of this study were to develop a population pharmacokinetic model for allopurinol and oxypurinol and to explore the influence of patient characteristics on allopurinol and oxypurinol pharmacokinetics.
Methods
Data from 92 patients with gout and 12 healthy volunteers were available for analysis. A parent–metabolite model with a two-compartment model for allopurinol and a one-compartment model for oxypurinol was fitted to the data using non-linear mixed effects modelling.
Results
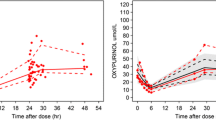
Renal function, fat-free mass (FFM) and diuretic use were found to predict differences in the pharmacokinetics of oxypurinol. The population estimates for allopurinol clearance, inter-compartmental clearance, central and peripheral volume were 50, 142 L/h/70 kg FFM, 11.4, 91 L/70 kg FFM, respectively, with a between-subject variability of 33 % (coefficient of variance, CV) for allopurinol clearance. Oxypurinol clearance and volume of distribution were estimated to be 0.78 L/h per 6 L/h creatinine clearance/70 kg FFM and 41 L/70 kg FFM in the final model, with a between-subject variability of 28 and 15 % (CV), respectively.
Conclusions
The pharmacokinetic model provides a means of predicting the allopurinol dose required to achieve target oxypurinol plasma concentrations for patients with different magnitudes of renal function, different body mass and with or without concomitant diuretic use. The model provides a basis for the rational dosing of allopurinol in clinical practice.




Similar content being viewed by others
References
Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P et al (2006) EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 65:1312–1324
Shoji A, Yamanaka H, Kamatani N (2004) A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum 51:321–325
Day RO, Graham GG, Hicks M, McLachlan AJ, Stocker SL, Williams KM (2007) Clinical pharmacokinetics and pharmacodynamics of allopurinol and oxypurinol. Clin Pharmacokinet 46:623–644
Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J et al (2007) British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology 46:1372–1374
Hande KR, Noone RM, Stone WJ (1984) Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med 76:47–56
US National Library of Medicine DailyMed: FDA information: allopurinol tablet (Zyloprim).Available at: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13564. Accessed 15 Aug 2012
Dalbeth N, Kumar S, Stamp L, Gow P (2006) Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol 33:1646–1650
Vazquez-Mellado J, Morales EM, Pacheco-Tena C, Burgos-Vargas R (2001) Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis 60:981–983
Stamp L, Gow P, Sharples K, Raill B (2000) The optimal use of allopurinol: an audit of allopurinol use in South Auckland. Aust NZ J Med 30:567–572
Stamp LK, Barclay ML, O’Donnell JL, Zhang M, Drake J, Frampton C, Chapman PT (2011) Relationship between serum urate and plasma oxypurinol in the management of gout: determination of minimum plasma oxypurinol concentration to achieve a target serum urate level. Clin Pharmacol Ther 90:392–398
Stamp LK, O’Donnell JL, Zhang M, James J, Frampton C, Barclay ML, Chapman PT (2011) Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum 63:412–421
Stamp LK, Barclay ML, O’Donnell JL, Zhang M, Drake J, Frampton C, Chapman PT (2012) Furosemide increases plasma oxypurinol without lowering serum urate—a complex drug interaction: implications for clinical practice. Rheumatology 51:1670–1676
Appelbaum SJ, Mayersohn M, Dorr RT, Perrier D (1982) Allopurinol kinetics and bioavailability. Intravenous, oral and rectal administration. Cancer Chemother Pharmacol 8:93–98
Breithaupt H, Tittel M (1982) Kinetics of allopurinol after single intravenous and oral doses: noninteraction with benzobromarone and hydrochlorothiazide. Eur J Clin Pharmacol 22:77–84
Boeckmann AJ, Sheiner LB, Beal SL (2010) NONMEM users guide—part VIII. ICON Development Solutions, Ellicott City
Zhang L, Beal SL, Sheiner LB (2003) Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn 30:387–404
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B (2005) Quantification of lean bodyweight. Clin Pharmacokinet 44:1051–1065
Anderson BJ, Holford NHG (2009) Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 24:25–36
Anderson BJ, Holford NH (2008) Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Stocker SL, Graham GG, McLachlan AJ, Williams KM, Day RO (2011) Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in patients with gout. J Rheumatol 38:904–910
Reyes AJ (2003) Cardiovascular drugs and serum uric acid. Cardiovasc Drugs Ther 17:397–414
Choi HK, Soriano LC, Zhang Y, Rodriguez LAG (2012) Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case–control study. BMJ 344:d8190
Daskalopoulou SS, Tzovaras V, Mikhailidis DP, Elisaf M (2005) Effect on serum uric acid levels of drugs prescribed for indications other than treating hyperuricaemia. Curr Pharm Des 11:4161–4175
Graessler J, Graessler A, Unger S, Kopprasch S, Tausche A-K, Kuhlisch E, Schroeder HE (2006) Association of the human urate transporter 1 with reduced renal uric acid excretion and hyperuricemia in a German Caucasian population. Arthritis Rheum 54:292–300
Stark K, Reinhard W, Grassl M, Erdmann J, Schunker H, Illig T, Hengstenberg C (2009) Common polymorphisms influencing serum uric acid levels contribute to susceptibility to gout, but not to coronary artery disease. PLoS One 4:e7729
van der Harst P, Bakker SJL, de Boer RA, Wolffenbuttel BHR, Johnson T, Caulfield MJ, Navis G (2010) Replication of the five novel loci for uric acid concentrations and potential mediating mechanisms. Hum Mol Genet 19:387–395
Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M et al (2009) Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 5:e1000504
Hollis-Moffatt JE, Xu X, Dalbeth N, Merriman ME, Topless R, Waddell C, Gow PJ, Harrison AA, Highton J, Jones PBB, Stamp LK, Merriman TR (2009) Role of the urate transporter SLC2A9 gene in susceptibility to gout in New Zealand Maori, Pacific Island, and Caucasian case–control sample sets. Arthritis Rheum 60:3485–3492
Hollis-Moffatt JE, Phipps-Green AJ, Chapman B, Jones GT, van Rij A, Gow PJ, Harrison AA, Highton J, Jones PB, Montgomery GW, Stamp LK, Dalbeth N, Merriman TR (2012) The renal urate transporter SLC17A1 locus: confirmation of association with gout. Arthritis Res 14:R92
Phipps-Green AJ, Hollis-Moffatt JE, Dalbeth N, Merriman ME, Topless R, Gow PJ et al (2010) A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Maori, case and control sample sets. Hum Mol Genet 19:4813–4819
Roberts RL, Zhang M, Marinaki AM, Stamp LK (2010) Does genetic variability in aldehyde oxidase and molybdenum cofactor sulfurase predict nonresponse to allopurinol? Aliment Pharmacol Ther 32:310–311
Smith MA, Marinaki AM, Arenas M, Shobowale-Bakre M, Lewis CM, Ansari A, Duley J, Sanderson D (2009) Novel pharmacogenetic markers for treatment outcome in azathioprine-treated inflammatory bowel disease. Aliment Pharmacol Ther 30:375–384
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151
Ahn JE, Karlsson MO, Dunne A, Ludden TM (2008) Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn 35:401–421
Stocker SL, McLachlan AJ, Savic RM, Kirkpatrick CM, Graham GG, Williams KM, Day RO (2012) The pharmacokinetics of oxypurinol in people with gout. Br J Clin Pharmacol 74:477–489
Day RO, Miners JO, Birkett DJ, Whitehead A, Naidoo D, Hayes J, Savdie E (1988) Allopurinol dose selection: relationships between dose and plasma oxypuirnol and serum urate concentrations and urinary rate excretion. Br J Clin Pharmacol 26:423–428
Peterson GM, Boyle RR, Francis HW, Oliver NWJ, Paterson J, von Witt RJ, Taylor GR (1990) Dosage prescribing and plasma oxypurinol levels in patients receiving allopurinol therapy. Eur J Clin Pharmacol 39:419–421
Graham S, Day RO, Wong H, McLachlan AJ, Bergendal L, Minors JO, Birkett DJ (1996) Pharmacodynamics of oxypurinol after administration of allopurinol to healthy subjects. Br J Clin Pharmacol 41:299–304
Delbarre F, Amor B, Auscher C, de Gery A (1966) Treatment of gout with allopurinol. A study of 106 cases. Ann Rheum Dis 25[6 Suppl]:627–633
Ta-F Y, Gutman AB (1964) Effect of allopurinol (4-Hydroxypyrazolo-(3,4-d)pyrimidine) on serum and urinary uric acid in primary and secondary gout. Am J Med 37:885–898
Klinenberg JR, Goldfinger SE, Seegmiller JE (1965) The effectiveness of the xanthine oxidase inhibitor allopurinol in the treatment of gout. Ann Intern Med 62:639
Rundles RW, Metz EN, Silberman HR (1966) Allopurinol in the treatment of gout. Ann Intern Med 64:229
Acknowledgments
At the time of writing, Dan Wright was the recipient of a University of Otago PhD scholarship. We wish to thank Dr. Rebecca Roberts, Department of Surgical Sciences, University of Otago, Dunedin for generous assistance with the AOX1 and MOCOS genotypes. We are also grateful to Jill Drake, Department of Rheumatology, Immunology and Allergy, Christchurch Hospital, for valuable assistance with the Christchurch data.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, D.F.B., Stamp, L.K., Merriman, T.R. et al. The population pharmacokinetics of allopurinol and oxypurinol in patients with gout. Eur J Clin Pharmacol 69, 1411–1421 (2013). https://doi.org/10.1007/s00228-013-1478-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1478-8