Abstract
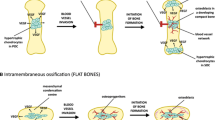
Adequate vascularization is an absolute requirement for bone development, growth, homeostasis, and repair. Endochondral ossification during fetal skeletogenesis is typified by the initial formation of a prefiguring cartilage template of the future bone, which itself is intrinsically avascular. When the chondrocytes reach terminal hypertrophic differentiation they become invaded by blood vessels. This neovascularization process triggers the progressive replacement of the growing cartilage by bone, in a complex multistep process that involves the coordinated activity of chondrocytes, osteoblasts, and osteoclasts, each standing in functional interaction with the vascular system. Studies using genetically modified mice have started to shed light on the molecular regulation of the cartilage neovascularization processes that drive endochondral bone development, growth, and repair, with a prime role being played by vascular endothelial growth factor and its isoforms. The vasculature of bone remains important throughout life as an intrinsic component of the bone and marrow environment. Bone remodeling, the continual renewal of bone by the balanced activities of osteoclasts resorbing packets of bone and osteoblasts building new bone, takes place in close spatial relationship with the vascular system and depends on signals, oxygen, and cellular delivery via the bloodstream. Conversely, the integrity and functionality of the vessel system, including the exchange of blood cells between the hematopoietic marrow and the circulation, rely on a delicate interplay with the cells of bone. Here, the current knowledge on the cellular relationships and molecular crosstalk that coordinate skeletal vascularization in bone development and homeostasis will be reviewed.



Similar content being viewed by others
References
Karsenty G, Wagner EF (2002) Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2:389–406
Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423:332–336
Trueta J, Morgan JD (1960) The vascular contribution to osteogenesis. I. Studies by the injection method. J Bone Joint Surg Br 42:97–109
Trueta J, Amato VP (1960) The vascular contribution to osteogenesis. III. Changes in the growth cartilage caused by experimentally induced ischaemia. J Bone Joint Surg Br 42B:571–587
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146:873–887
Ferrara N (2009) VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw 20:158–163
Colnot C, Lu C, Hu D, Helms JA (2004) Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol 269:55–69
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985
Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT (1989) Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 246:1309–1312
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309
Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J (2007) Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol 49:1015–1026
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signaling—in control of vascular function. Nat Rev Mol Cell Biol 7:359–371
Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N (2002) VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417:954–958
Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell 130:691–703
Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR (2012) Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest 122:3101–3113
Harper SJ, Bates DO (2008) VEGF-A splicing: The key to anti-angiogenic therapeutics? Nat Rev Cancer 8:880–887
Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT (2002) Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 16:2684–2698
Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, Bouillon R, Carmeliet G (2002) Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev 111:61–73
Dai J, Rabie AB (2007) VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res 86:937–950
Maes C, Carmeliet G, Schipani E (2012) Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol 8:358–366
Zelzer E, Glotzer D, Hartmann C, Thomas D, Fukai N, Soker S, Olsen B (2001) Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech Dev 106:97–106
Tang W, Yang F, Li Y, de Crombrugghe B, Jiao H, Xiao G, Zhang C (2012) Transcriptional regulation of vascular endothelial growth factor (VEGF) by osteoblast-specific transcription factor Osterix (Osx) in osteoblasts. J Biol Chem 287:1671–1678
Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N (1999) VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5:623–628
Haigh JJ, Gerber HP, Ferrara N, Wagner EF (2000) Conditional inactivation of VEGF-A in areas of collagen2a1 expression results in embryonic lethality in the heterozygous state. Development 127:1445–1453
Maes C, Stockmans I, Moermans K, Looveren RV, Smets N, Carmeliet P, Bouillon R, Carmeliet G (2004) Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest 113:188–199
Zelzer E, Mamluk R, Ferrara N, Johnson R, Schipani E, Olsen B (2004) VEGFA is necessary for chondrocyte survival during bone development. Development 131:2161–2171
Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S, D’Amore PA, Olsen BR (2002) Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 129:1893–1904
Eshkar-Oren I, Viukov SV, Salameh S, Krief S, Oh CD, Akiyama H, Gerber HP, Ferrara N, Zelzer E (2009) The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development 136:1263–1272
Gerber HP, Ferrara N (2000) Angiogenesis and bone growth. Trends Cardiovasc Med 10:223–228
Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z (1998) MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411–422
Krane SM, Inada M (2008) Matrix metalloproteinases and bone. Bone 43:7–18
Ishijima M, Suzuki N, Hozumi K, Matsunobu T, Kosaki K, Kaneko H, Hassell JR, Arikawa-Hirasawa E, Yamada Y (2012) Perlecan modulates VEGF signaling and is essential for vascularization in endochondral bone formation. Matrix Biol 31:234–245
Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar VV, Stalmans I, Mattot V, Perriard JC, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D’Amore PA, Shima DT (1999) Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med 5:495–502
Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM (2000) Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol 151:879–889
Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G (2006) Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts 2. J Clin Invest 116:3150–3159
Takamoto M, Tsuji K, Yamashita T, Sasaki H, Yano T, Taketani Y, Komori T, Nifuji A, Noda M (2003) Hedgehog signaling enhances core-binding factor a1 and receptor activator of nuclear factor-kappa B ligand (RANKL) gene expression in chondrocytes. J Endocrinol 177:413–421
Aldridge SE, Lennard TW, Williams JR, Birch MA (2005) Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem Biophys Res Commun 335:793–798
Nakagawa M, Kaneda T, Arakawa T, Morita S, Sato T, Yomada T, Hanada K, Kumegawa M, Hakeda Y (2000) Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts 4. FEBS Lett 473:161–164
Niida S, Kaku M, Amano H, Yoshida H, Kataoka H, Nishikawa S, Tanne K, Maeda N, Kodama H (1999) Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption 2. J Exp Med 190:293–298
Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z (2004) Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 131:5883–5895
Gartland A, Mason-Savas A, Yang M, MacKay CA, Birnbaum MJ, Odgren PR (2009) Septoclast deficiency accompanies postnatal growth plate chondrodysplasia in the toothless (tl) osteopetrotic, colony-stimulating factor-1 (CSF-1)-deficient rat and is partially responsive to CSF-1 injections. Am J Pathol 175:2668–2675
Lee ER, Lamplugh L, Shepard NL, Mort JS (1995) The septoclast, a cathepsin B-rich cell involved in the resorption of growth plate cartilage. J Histochem Cytochem 43:525–536
Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM (2010) Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 19:329–344
Poulton IJ, McGregor NE, Pompolo S, Walker EC, Sims NA (2012) Contrasting roles of leukemia inhibitory factor in murine bone development and remodeling involve region-specific changes in vascularization. J Bone Miner Res 27:586–595
Ortega N, Wang K, Ferrara N, Werb Z, Vu TH (2010) Complementary interplay between matrix metalloproteinase-9, vascular endothelial growth factor and osteoclast function drives endochondral bone formation. Dis Model Mech 3:224–235
Van Wesenbeeck L, Odgren PR, MacKay CA, D’Angelo M, Safadi FF, Popoff SN, Van Hul W, Marks SC Jr (2002) The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. Proc Natl Acad Sci USA 99:14303–14308
Day T, Guo X, Garrett-Beal L, Yang Y (2005) Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750
Hill T, Spater D, Taketo M, Birchmeier W, Hartmann C (2005) Canonical wnt/betacatenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8:727–738
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng J, Behringer R, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29
Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ (1997) Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771
St Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13:2072–2086
Kronenberg HM (2007) The role of the perichondrium in fetal bone development. Ann N Y Acad Sci 1116:59–64
Komori T (2011) Signaling networks in RUNX2-dependent bone development. J Cell Biochem 112:750–755
Long F (2012) Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 13:27–38
Chung UI, Schipani E, McMahon AP, Kronenberg HM (2001) Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest 107:295–304
Takeda S, Bonnamy JP, Owen MJ, Ducy P, Karsenty G (2001) Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev 15:467–481
Fiedler J, Etzel N, Brenner RE (2004) To go or not to go: migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J Cell Biochem 93:990–998
Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Gunther KP, Dehio C, Puhl W, Brenner RE (2002) Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone 30:472–477
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X (2009) TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15:757–765
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313
Modder UI, Khosla S (2008) Skeletal stem/osteoprogenitor cells: current concepts, alternate hypotheses, and relationship to the bone remodeling compartment. J Cell Biochem 103:393–400
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131:324–336
Kalajzic Z, Li H, Wang LP, Jiang X, Lamothe K, Adams DJ, Aguila HL, Rowe DW, Kalajzic I (2008) Use of an alpha-smooth muscle actin GFP reporter to identify an osteoprogenitor population. Bone 43:501–510
Yin M, Pacifici M (2001) Vascular regression is required for mesenchymal condensation and chondrogenesis in the developing limb. Dev Dyn 222:522–533
Shukunami C, Oshima Y, Hiraki Y (2005) Chondromodulin-I and tenomodulin: a new class of tissue-specific angiogenesis inhibitors found in hypovascular connective tissues. Biochem Biophys Res Commun 333:299–307
Brandau O, Aszodi A, Hunziker EB, Neame PJ, Vestweber D, Fassler R (2002) Chondromodulin I is dispensable during enchondral ossification and eye development. Mol Cell Biol 22:6627–6635
Wirth T, Syed Ali MM, Rauer C, Suss D, Griss P, Syed Ali S (2002) The blood supply of the growth plate and the epiphysis: a comparative scanning electron microscopy and histological experimental study in growing sheep. Calcif Tissue Int 70:312–319
Maes C, Araldi E, Haigh K, Khatri R, Van Looveren R, Giaccia AJ, Haigh JJ, Carmeliet G, Schipani E (2012) VEGF-independent cell-autonomous functions of HIF-1alpha regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J Bone Miner Res 27:596–609
Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K (2000) Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA 97:4052–4057
Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS (2001) Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev 15:2865–2876
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408
Cramer T, Schipani E, Johnson RS, Swoboda B, Pfander D (2004) Expression of VEGF isoforms by epiphyseal chondrocytes during low-oxygen tension is HIF-1 alpha dependent. Osteoarthr Cartil 12:433–439
Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL (2007) The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117:1616–1626
Riddle RC, Khatri R, Schipani E, Clemens TL (2009) Role of hypoxia-inducible factor-1alpha in angiogenic–osteogenic coupling. J Mol Med 87:583–590
Provot S, Zinyk D, Gunes Y, Kathri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ, Schipani E (2007) HIF-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol 177:451–464
Pfander D, Cramer T, Schipani E, Johnson R (2003) HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci 116:1819–1826
Lafage-Proust MH, Prisby R, Roche B, Vico L (2010) Bone vascularization and remodeling. Joint Bone Spine 77:521–524
Andersen TL, Sondergaard TE, Skorzynska KE, Dagnaes-Hansen F, Plesner TL, Hauge EM, Plesner T, Delaisse JM (2009) A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol 174:239–247
Schipani E, Maes C, Carmeliet G, Semenza GL (2009) Regulation of osteogenesis–angiogenesis coupling by HIFs and VEGF. J Bone Miner Res 24:1347–1353
Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert S, Bouxsein M, Faugere M, Guldberg R, Gerstenfeld L, Haase V, Johnson R, Schipani E, Clemens T (2007) The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117:1616–1626
Maes C, Goossens S, Bartunkova S, Drogat B, Coenegrachts L, Stockmans I, Moermans K, Nyabi O, Haigh K, Naessens M, Haenebalcke L, Tuckermann JP, Tjwa M, Carmeliet P, Mandic V, David JP, Behrens A, Nagy A, Carmeliet G, Haigh JJ (2010) Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J 29:424–441
Guerrouahen BS, Al-Hijji I, Tabrizi AR (2011) Osteoblastic and vascular endothelial niches, their control on normal hematopoietic stem cells, and their consequences on the development of leukemia. Stem Cells Int 2011:375857
Coskun S, Hirschi KK (2010) Establishment and regulation of the HSC niche: roles of osteoblastic and vascular compartments. Birth Defects Res C 90:229–242
Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121
Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK, Luster AD, Scadden DT, Lin CP (2005) In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435:969–973
Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, Rafii S (2010) Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol 12:1046–1056
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834
Sugiyama T, Kohara H, Noda M, Nagasawa T (2006) Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25:977–988
Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT (2009) Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457:92–96
Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC, Park J, Haug JS, Wunderlich JP, Li H, Zhang S, Johnson T, Feldman RA, Li L (2009) Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 457:97–101
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425:836–841
Kiel MJ, Acar M, Radice GL, Morrison SJ (2009) Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell 4:170–179
Bromberg O, Frisch BJ, Weber JM, Porter RL, Civitelli R, Calvi LM (2012) Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood 120:303–313
Greenbaum AM, Revollo LD, Woloszynek JR, Civitelli R, Link DC (2012) N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood 120:295–302
Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA (2010) The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7:380–390
Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, Araldi E, Rankin AL, Yuan J, Kuo CJ, Schipani E, Giaccia AJ (2012) The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149:63–74
Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, Westmoreland SV, Chambon P, Scadden DT, Purton LE (2007) A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 129:1097–1110
Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT (2010) Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464:852–857
Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B, Mundy GR (2008) Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood 111:2833–2842
Street J, Winter D, Wang JH, Wakai A, McGuinness A, Redmond HP (2000) Is human fracture hematoma inherently angiogenic? Clin Orthop Relat Res 378:224–237
Beamer B, Hettrich C, Lane J (2010) Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing. HSS J 6:85–94
Schindeler A, McDonald MM, Bokko P, Little DG (2008) Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol 19:459–466
Kumar S, Ponnazhagan S (2012) Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone 50:1012–1018
Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA (2003) Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 88:873–884
Ferguson CM, Miclau T, Hu D, Alpern E, Helms JA (1998) Common molecular pathways in skeletal morphogenesis and repair. Ann N Y Acad Sci 857:33–42
Jacobsen KA, Al Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, Einhorn TA, Gerstenfeld LC (2008) Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res 23:596–609
Street J, Bao M, de Guzman L, Bunting S, Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA 99:9656–9661
Behonick DJ, Xing Z, Lieu S, Buckley JM, Lotz JC, Marcucio RS, Werb Z, Miclau T, Colnot C (2007) Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration. PLoS One 2:e1150
Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA (2003) Altered fracture repair in the absence of MMP9. Development 130:4123–4133
Lieu S, Hansen E, Dedini R, Behonick D, Werb Z, Miclau T, Marcucio R, Colnot C (2011) Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis Model Mech 4:203–211
Colnot C (2009) Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 24:274–282
Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, Longaker MT (2002) Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg 109:2384–2397
Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV (2008) Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury 39(Suppl 2):S45–S57
Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S (2010) Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther 18:1026–1034
Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J (2002) Synergistic enhancement of bone formation and healing by stem cell–expressed VEGF and bone morphogenetic protein-4. J Clin Invest 110:751–759
Acknowledgments
The author acknowledges all colleagues she had many fruitful discussions with. Special thanks goes to Dr. Natalie Sims for providing thoughtful comments on the manuscript. The financial support from Starting Grant 282131 from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007–2013) is gratefully acknowledged. The author sincerely apologizes to all those whose work was not included due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Additional information
The author has stated that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Maes, C. Role and Regulation of Vascularization Processes in Endochondral Bones. Calcif Tissue Int 92, 307–323 (2013). https://doi.org/10.1007/s00223-012-9689-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-012-9689-z